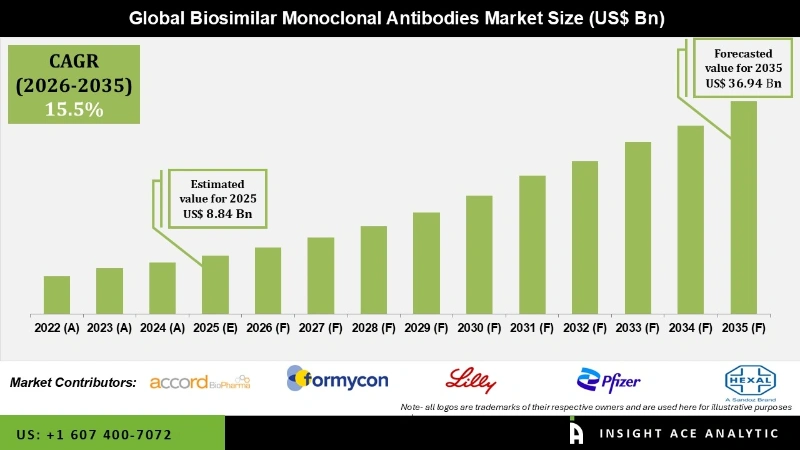
Global Biosimilar Monoclonal Antibodies Market Size is valued at USD 8.84 Billion in 2025 and is predicted to reach USD 36.94 Billion by the year 2035 at a 15.5% CAGR during the forecast period for 2026 to 2035.
Biosimilar Monoclonal Antibodies Market Size, Share & Trends Analysis Report By Product (Infliximab, Trastuzumab, Rituximab, Adalimumab, Bevacizumab, Cetuximab, Ranibizumab, Denosumab, Eculizumab, Other Pipeline Products), By Indication, By Region, And Segment Forecasts, 2026 to 2035.
Key Industry Insights & Findings from the Report:
Biosimilars are biological drugs, and they are large, complex biological molecules produced from living organisms that are often difficult to characterize and therefore difficult to copy. Monoclonal antibody biosimilars play a vital role in treating various chronic and autoimmune diseases. They also represent an opportunity to increase access and reduce costs for patients and healthcare systems.
Global Biosimilar Monoclonal Antibodies market growth is attributed to numerous factors such as biosimilar monoclonal antibodies market include the increasing patent expiries of mAbs, innovation of advanced mAbs, the growing number of biosimilars for oncology treatments, rising demand for lower-priced, cost-efficient biosimilar monoclonal antibodies, growing chronic and autoimmune diseases like cancer, rheumatoid arthritis, diabetes, and the rising older population. Moreover, increasing R&D investments for biological drugs are anticipated to fuel market adoption during the forecast period. Growing R&D activities result in innovative drug products, developmental procedures, and healthcare facilities, thereby boosting market growth.
However, the high cost of development processes, complex mAb manufacturing procedures, region-specific government rules regarding the production and use of biosimilars, lack of scientific standards for biosimilars are expected to hamper the market's growth in the coming years.
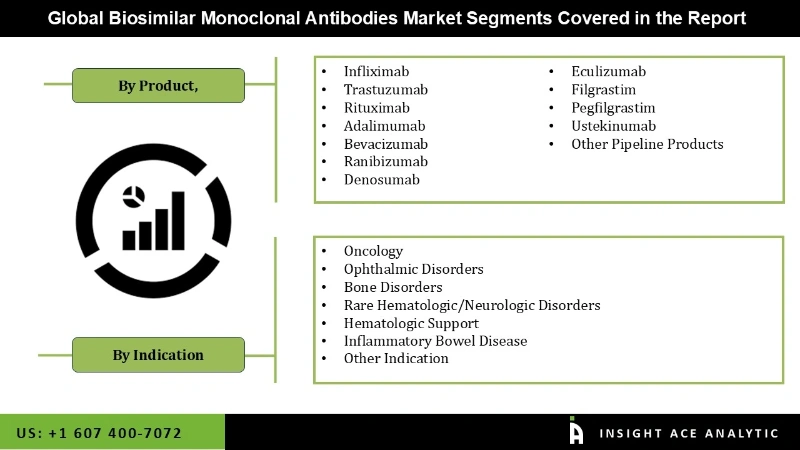
The Biosimilar Monoclonal Antibodies Market is segmented based on product, indication, and region. By product, the market comprises the infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products. The infliximab and rituximab segments are projected to hold the highest market share over the forecast period owing to their increasing use in treating various chronic and autoimmune diseases. The market is classified into oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications based on the indication. Among all, The oncology segment is predictable to dominate the market in the coming years due to the surge in biosimilar mAbs approvals for cancer treatments. Regionally, the market is categorized across North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
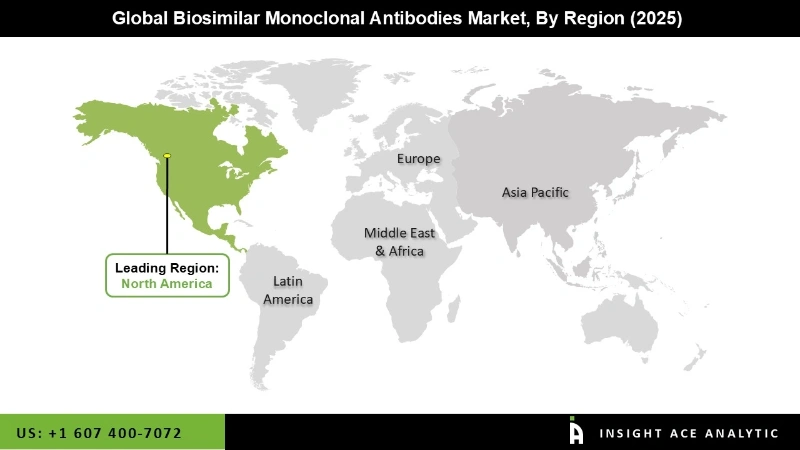
North America region dominated this market in 2020 and is expected to continue its trend over the forecast period on account of the favourable reimbursement policies, increasing patent expiration, and the growing rate of biosimilar mAbs approvals.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 8.84 Billion |
| Revenue Forecast In 2035 | USD 36.94 Billion |
| Growth Rate CAGR | CAGR of 15.5% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn, and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Products, By Indication |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
| Competitive Landscape | Pfizer, Novartis, Allergan, Coherus BioSciences, Biocon, Amgen, Boehringer Ingelheim, Celltrion, BioXpress Therapeutics, Intas Pharmaceuticals Limited, Genor BioPharma Co., Ltd, BIOCAD, Reddys Laboratories Ltd, 3SBio, Reliance Life Sciences, Hisun Pharma, Celgen Biopharma, Torrent Pharmaceuticals, Cadila Healthcare, Mylan Inc., BioXpress Therapeutics SA, Celltrion, EirGenix, Inc., Teva Pharmaceutical Industries Ltd., ALTEOGEN Inc., Apotex (Apobiologix), AryoGen Pharmed, Prestige BioPharma (PBP), PlantForm Outlook Therapeutics (Oncobiologics), Shanghai CP Guojian Pharmaceutical, Shanghai Henlius Biotech, Stada Arzneimittel AG, Other Prominent Players |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Biosimilar Monoclonal Antibodies Market, by Product,
Global Biosimilar Monoclonal Antibodies Market, by Indication, Autoimmune Diseases
Global Biosimilar Monoclonal Antibodies Market, by Region,
North America
Europe
Asia Pacific Biosimilar
Latin America
Middle East & Africa
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.