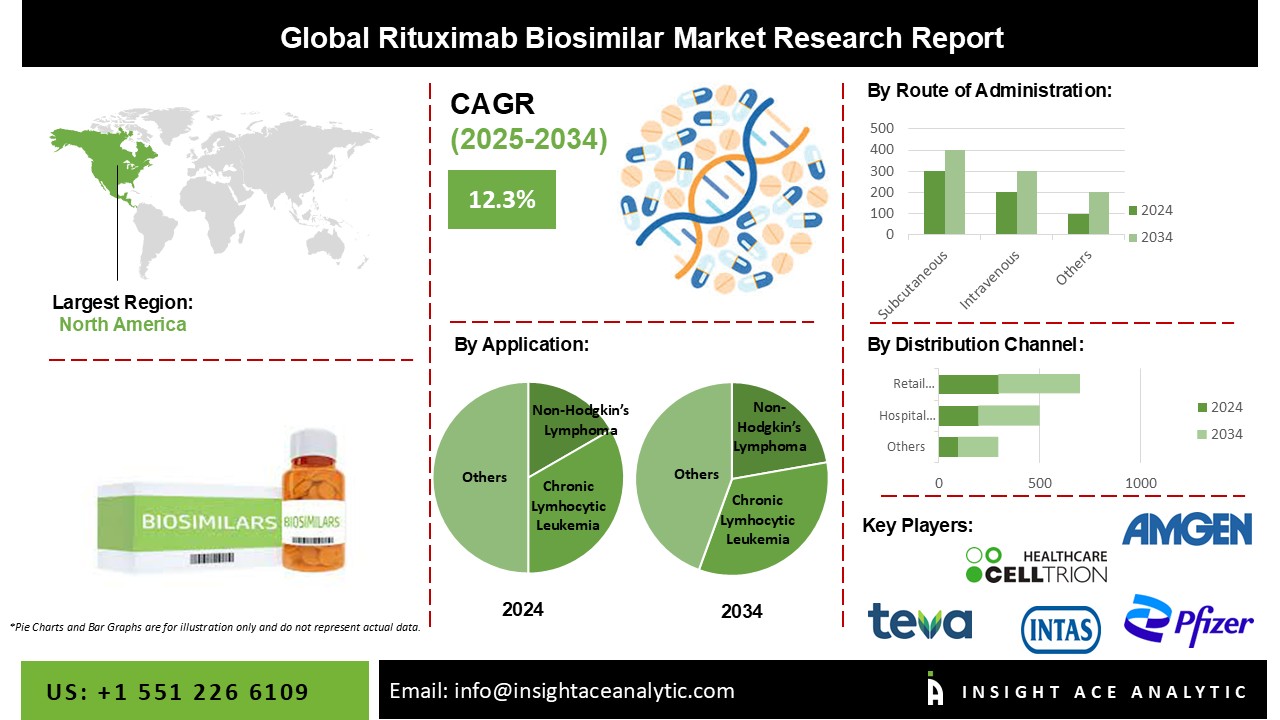
Rituximab Biosimilar Market is expected to grow at a 12.3% CAGR during the forecast period for 2025-2034.
Rituximab is a chimeric anti-CD20 antibody that binds to the CD20 antigen present on the standard and malignant B cells surface. It is indicated for the treatment of blood cancer (CD20-positive) such as chronic lymphocytic leukemia and non-Hodgkin’s lymphoma, as well as an autoimmune disorder such as Rheumatoid arthritis. Rituximab is marketed under the trade name of MabThera in Europe and the Rest of the World, except Japan by Roche. In the United States, it is marketed as Rituxan by Genentech in partnership with Biogen Idec. In Japan, it is marketed by Chugai and Zenyaku Kogyo Co. Ltd. It was approved in the U.S. in 1997 and by European Medical Agency in June 1998. Rituximab is one of the top selling pharmaceutical products of Roche Holding AG. Therefore many biosimilar pharmaceutical companies are focusing on product development of rituximab biosimilar to compel the biosimilar product market share.
The rituximab biosimilar market is expected to grow at a higher pace during the forecast period since the rituximab is one of the best seller drug products that accounts for around 2.1 million prescriptions worldwide since the market launch. In addition, rituximab has lost its patent in the United States and Europe in 2016 and 2013, respectively. The presence of strong pipeline products such as ABP 789, MabTas, Redux, and JHL 101, increasing incidence of chronic disease (such as cancer, arthritis, and diabetes) are the other factor that boosts the demand for the rituximab biosimilar market. Furthermore, rising demand for biosimilar drugs and the entry of new pharmaceutical market players have increased the growth of the rituximab biosimilar market during the forecast period. However, a stringent regulatory approval process for the rituximab biosimilar is a major restraining factor that hampers the growth of the rituximab biosimilar market. In addition, IP strategies used by the product innovator companies to fight against the biosimilar competition, such as advancement in original formulation or screening for some new indications, restrain the growth of the rituximab biosimilar market. For instance, Roche announced the development of a new formulation for Rituxan as a strategy to fight against biosimilar competition.
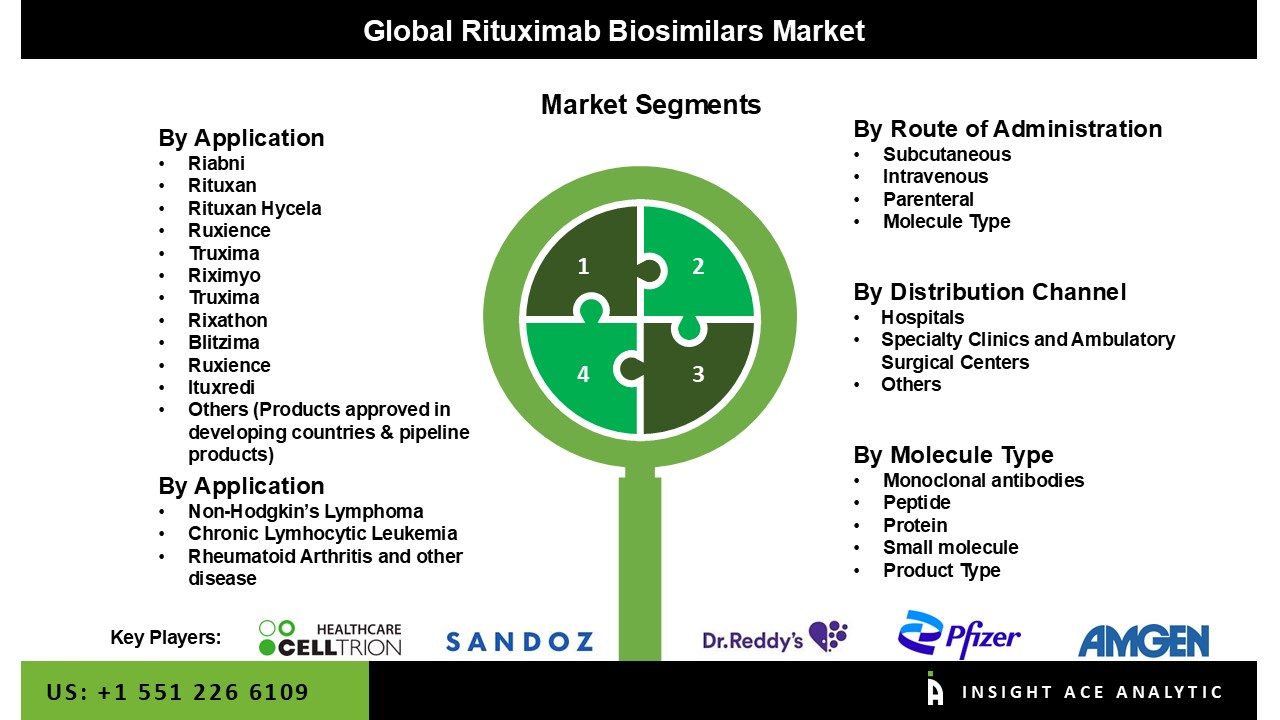
As per the brand, the makret consists of Riabni, Rituxan, Rituxan Hycela, Ruxience, Truxima, Riximyo, Rixathon, Blitzima, Ituxredi, and others (including products approved in developing countries and pipeline products). Based on application, the global rituximab biosimilar market is segmented into non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, and others. Other indication includes off-label use such as thrombocytopenia, macroglobulinemia, immune thrombocytopenic purpura, Burkitt lymphoma, autoimmune hemolytic anemia, and multiple sclerosis. Non-Hodgkin’s lymphoma and chronic lymphocytic leukemia segment are expected to grow at a rapid pace during the forecast period owing to the increasing incidence and prevalence rate of chronic disease. Based on distribution channels, the global rituximab biosimilar market is segmented as hospital pharmacy, online pharmacy, retail pharmacy, and others. The hospital pharmacy segment is expected to grow during the forecast period due to an increase in the number of hospital visits and hospital stays for the treatment of different chronic diseases.
At geographic level segmentation, the global rituximab biosimilar market has been categorized into Europe, Asia Pacific, North America, Latin America, and Middle East & Africa. In terms of revenue, Europe is a major contributor to the global rituximab biosimilar market. Europe is the major contributor to the global market owing to the loss of a rituximab patent in 2013. In June 2017, Sandoz announced the launch of the first rituximab biosimilar in Europe. Europe is followed by North America, which is the second major revenue generator during the forecast period. The growth of the rituximab biosimilar market in North America is owing to factors such as strong clinical trial/product pipeline, increasing research and drug development activities propels rituximab biosimilar market growth in the region. Asia Pacific is the third promising revenue contributor which is expected to grow at rapid pace in the upcoming year. Countries such as Japan, India, and China are major contributors to this market. Emerging and huge population base countries such as China and India offer tremendous market opportunities.
| Report Attribute | Specifications |
| Growth Rate CAGR | CAGR of 12.3% from 2025 to 2034 |
| Quantitative Units | Representation of revenue in US$ Million and CAGR from 2025 to 2034 |
| Historic Year | 2021 to 2024 |
| Forecast Year | 2025-2034 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Brand, By Application, By Route of Administration, By Molecule Type, By Distribution Channel |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Amgen Inc., Pfizer Inc., Sandoz International GmbH, Celltrion Healthcare Co., Ltd., Mylan Inc., Reliance Life Sciences, Teva Pharmaceutical Industries Ltd., Intas Biopharmaceuticals Ltd., C.H. Boehringer Sohn AG & Ko. KG, and BioXpress Therapeutics SA. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Rituximab Biosimilar Market Outlook By Brand
Global Rituximab Biosimilar Market Outlook By Application
Global Rituximab Biosimilar Market Outlook By Route of Administration
Global Rituximab Biosimilar Market Outlook By Molecule Type
Global Rituximab Biosimilar Market Outlook By Distribution Channel
Global Rituximab Biosimilar Market Outlook By Region
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.