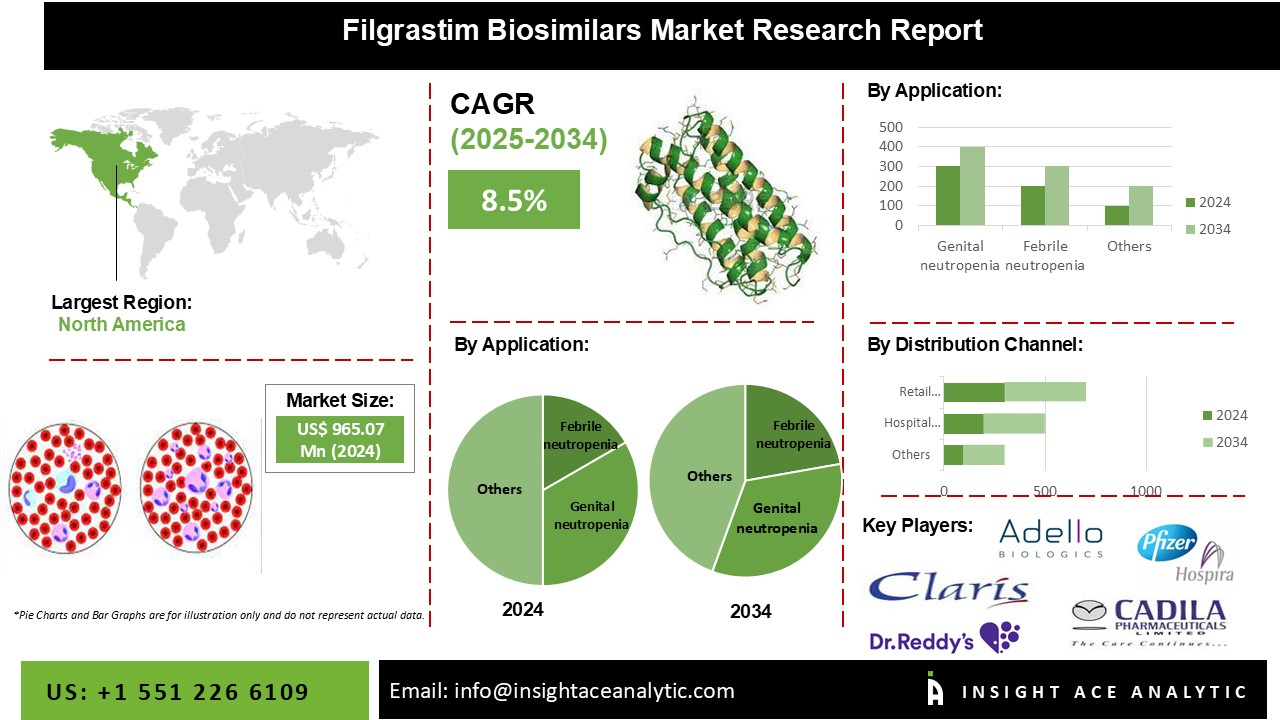
Filgrastim Biosimilars Market Size is predicted to expand with a 8.5% CAGR during the forecast period for 2025-2034.
Key Industry Insights & Findings from the Report:
Myeloid growth factor therapy has become the standard therapy for the treatment of neutropenia. The myeloid-specific cytokine G-CSF (granulocyte-colony stimulating factor) is the primary growth factor that increases neutrophil production. The human G-CSF gene was transduced and incorporated into Escherichia coli through recombinant DNA technology, formed drug is known as ‘filgrastim’. Filgrastim is G-CSF that stimulates the bone marrow to produce more neutrophils. The drug was developed by Amgen and marketed under the trade name Neupogen. It was approved in the U.S. by Food Drug and Administration in February 1991. Neupogen is approved for various indications, such as febrile neutropenia. It is indicated to reduce the recovery time of neutrophils and the duration of fever following chemotherapy treatment of patients suffering from acute myeloid leukemia. For the patient who are undergoing myeloablative chemotherapy in nonmyeloid malignancies, filgrastim reduces the duration of neutropenia and neutropenia-related clinical sequelae. In addition, it is used as a stem cell mobilizer and for the treatment of cyclic‚ genital‚ or idiopathic neutropenia.
The patents on Neupogen expired in the US in December 2013 and in Europe in 2006. The entry of filgrastim biosimilar into the market such as Zarzio (Sandoz), Nufil (Biocon), Grastofil (Apotex) and many more have boosted the growth of the filgrastim biosimilar market. Growing demand for biosimilar and increase in demand for cost effective treatment in low and medium-income countries further drives the growth of the filgrastim biosimilar market during the forecast period. Moreover, approximately half of cancer patients receiving chemotherapy experience some type of neutropenia, thus increase the demand for filgrastim biosimilars. However, stringent regulatory process for the approval of biosimilar and complicated manufacturing process is major restrain for the growth of the global filgrastim biosimilar market.
The global filgrastim market is segmented on the basis of application, distribution channel, and geography. On the basis of application, the global filgrastim market is segmented into febrile neutropenia, genital neutropenia, idiopathic neutropenia, and many more. An increase in the incidence of cancer patients undergoing chemotherapy has boosted the growth of the market. Based on distribution channel, the global filgrastim market is segmented into online pharmacy, hospital pharmacy and retail pharmacy. Hospital pharmacy is a major contributor in terms of revenue to the market due to increase in a number of hospital visits and hospital stays.
At the regional level, the global filgrastim biosimilar market has been segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. In terms of revenue, North America followed by Europe is a major contributor to the global filgrastim biosimilar market. This is partly because of increased government initiatives for cost-effective treatment and an increase in demand for biosimilars. Furthermore, a strong clinical pipeline and increasing research and drug development activities propel the growth of the market in these regions. Asia Pacific is the third promising revenue contributor which is expected to grow at rapid pace in the upcoming year. Countries such as Japan, India, and China are major contributors to this market and are identified as most lucrative for this market. Emerging and huge population base countries such as China and India offer tremendous market opportunities.
| Report Attribute | Specifications |
| Growth Rate CAGR | CAGR of 8.5% from 2025 to 2034 |
| Quantitative Units | Representation of revenue in US$ Million and CAGR from 2025 to 2034 |
| Historic Year | 2021 to 2024 |
| Forecast Year | 2025-2034 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Application, By Distribution Channel |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Aryogen Biopharma, Cadila Pharmaceutical, Claris Life Sciences, Dr Reddy’s Laboratories, Intas Biopharmaceuticals, Adello Biologics, Hospira(Pfizer), Genova Biopharmaceuticals (Emcure), Teva Pharmaceutical Industries, Sandoz, and others |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Filgrastim Biosimilars Market Outlook By Application
Global Filgrastim Biosimilars Market Outlook By Distribution Channel
Global Filgrastim Biosimilars Market Outlook By Region
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.