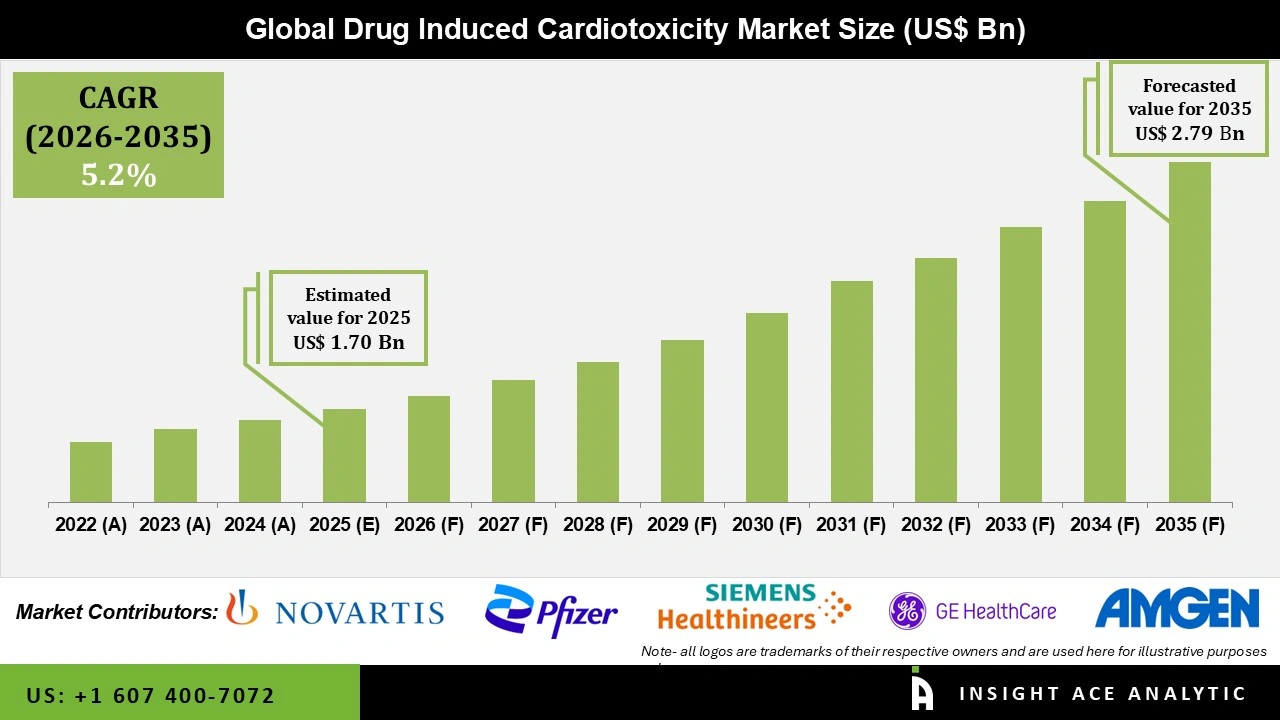
Global Drug-Induced Cardiotoxicity Market Size is valued at USD 1.70 Bn in 2025 and is predicted to reach USD 2.79 Bn by the year 2035 at a 5.2% CAGR during the forecast period for 2026 to 2035.
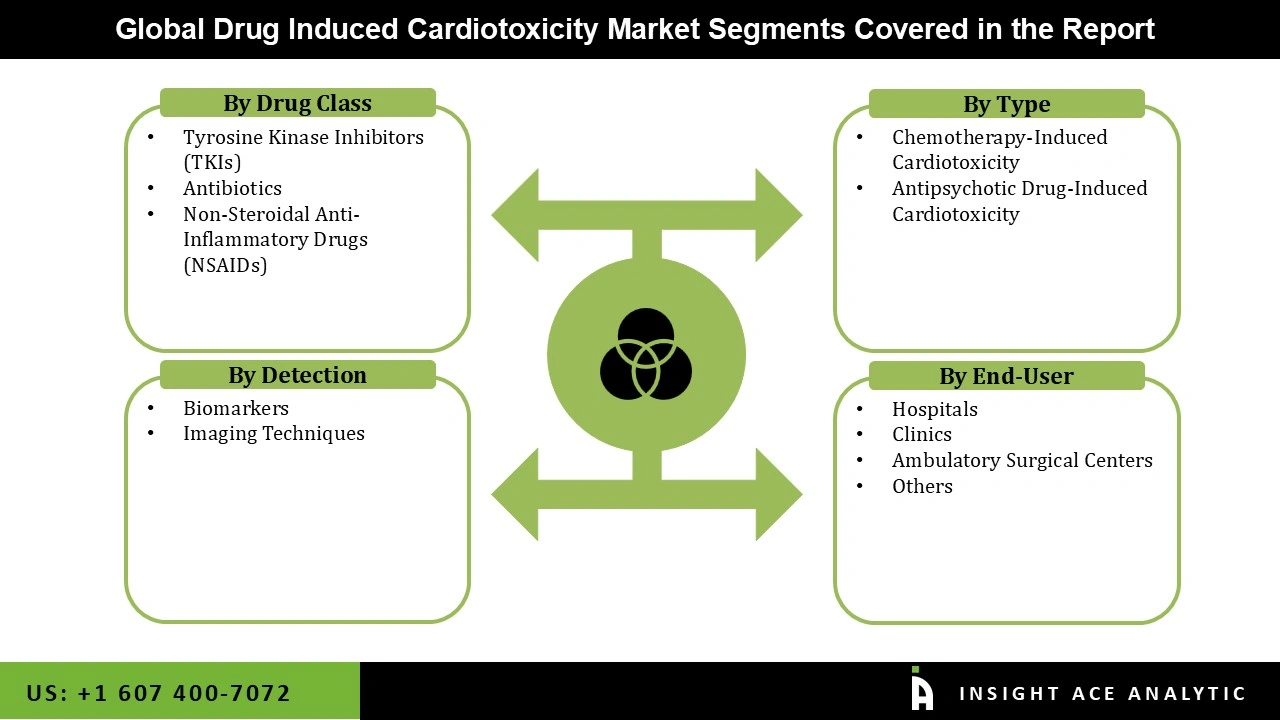
Drug-Induced Cardiotoxicity Market Size, Share & Trends Analysis Distribution by Type (Antipsychotic Drug-Induced Cardiotoxicity and Chemotherapy-Induced Cardiotoxicity), Drug Class (Antibiotics, Tyrosine Kinase Inhibitors (TKIs), and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)), Detection (Imaging Techniques and Biomarkers), End-user (Hospitals, Clinics, Ambulatory Surgical Centers, and Others), By Region and Segment Forecasts, 2026 to 2035.
Drug-induced cardiotoxicity refers to structural or functional damage to the myocardium and/or cardiovascular system resulting from exposure to pharmaceutical agents or therapeutic interventions. While various drug classes including certain antibiotics, antipsychotics, immunomodulators, and antiarrhythmic agents may contribute, the condition is most prominently associated with antineoplastic therapies, particularly anthracyclines (e.g., doxorubicin, daunorubicin), HER2-targeted monoclonal antibodies (e.g., trastuzumab), multi-targeted tyrosine kinase inhibitors, and immune checkpoint inhibitors.
The increasing clinical relevance of drug-induced cardiotoxicity reflects the expanding use of potentially cardiotoxic anticancer therapies and improved long-term survival of cancer patients, underscoring the need for robust risk stratification, preventive strategies, and multidisciplinary cardio-oncology management to balance oncologic efficacy with cardiovascular safety. The growing prevalence of cardiovascular problems linked to popular treatment classes like immunotherapies, targeted therapies, and cancer medications is the main factor propelling the drug-induced cardiotoxicity market.
Early diagnosis and monitoring procedures have been improved as a result of healthcare professionals' growing awareness of the cardiotoxic risk of medications like HER2 inhibitors and anthracyclines. The need for cardioprotective measures, cardiac monitoring systems, and efficient management treatments keeps increasing as cancer survival rates rise and patients go through extended treatment plans. Further contributing to the growing need for specific cardiotoxicity control solutions is the aging population, which is more susceptible to both chronic illnesses and adverse drug reactions.
Moreover, improvements in diagnostic modalities, such as sophisticated cardiac imaging techniques and biomarker-based screening, are improving early detection of cardiac dysfunction, facilitating prompt intervention and lowering long-term consequences. The market expansion is also being reinforced by the increasing expenditures made by biotechnology and pharmaceutical businesses in the development of safer medicine formulations and cardioprotective substances. The treatment environment is changing as a result of the combination of interdisciplinary cardio-oncology techniques, risk stratification technologies, and precision medicine.
Driver
Increasing Incidence of Cardiovascular Disorders Worldwide
One of the major factors propelling the drug-induced cardiotoxicity market is the increasing incidence of cardiovascular disorders worldwide. The necessity to monitor and manage these risks is highlighted by the rise in the use of medications that may have cardiotoxic effects as more people receive treatment for cardiovascular problems. This pattern highlights the increasing need for cardiotoxicity testing and monitoring services in order to avoid side effects from cardiovascular medications. According to the most recent data from the American College of Cardiology, fatalities from cardiovascular diseases (CVD) increased from 12.4 Mn in 1990 to 19.8 Mn in 2022, making them the top cause of death worldwide. The market's increasing need for drug-induced cardiotoxicity monitoring systems is highlighted by this noteworthy rise.
Restrain/Challenge
High Expense of Cardiotoxicity Testing
The drug-induced cardiotoxicity market is significantly hampered by the high expense of cardiotoxicity testing, which mostly affects smaller pharmaceutical companies and areas with tighter healthcare budgets. While testing methods like cardiac MRIs and echocardiograms are essential for keeping an eye on heart health throughout cancer therapy, they can be costly. The costs are further increased by the Cleveland Clinic, which emphasizes that managing cardiotoxicity requires numerous, frequent diagnostic procedures to track heart function. Comprehensive cardiotoxicity testing techniques are crucial for averting severe cardiac events in cancer patients receiving therapy, but their acceptance may be slowed by this financial burden. Therefore, the financial consequences of these tests may impede their extensive market adoption, especially for organizations with low financial resources.
The Chemotherapy-Induced Cardiotoxicity category held the largest share in the Drug-Induced Cardiotoxicity market in 2025. The rising incidence of cancer as well as the corresponding increase in cardiotoxicity cases are driving the continuous growth of the chemotherapy-induced cardiotoxicity (CIC) segment. The segment's growth is being boosted by the increased use of cardiotoxic chemotherapy medicines due to the rise in cancer rates worldwide. Additionally, the market is expanding due to a number of factors, such as the development of new therapeutic agents that specifically target cardiotoxicity, improvements in diagnostic methods that enable earlier detection of CIC, and a growing understanding among medical professionals of the significance of preventative measures and efficient management of chemotherapy-induced cardiotoxicity.
In 2025, the Tyrosine Kinase Inhibitors (TKIs) category dominated the Drug-Induced Cardiotoxicity market because targeted treatment and oncology have made extensive use of these agents. TKIs, including sunitinib, nilotinib, dasatinib, and imatinib, are widely used to treat gastrointestinal stromal tumors, renal cell carcinoma, chronic myeloid leukemia, and other cancers. Despite their great effectiveness, these medications are becoming more and more linked to cardiovascular side effects, including as heart failure, coronary thrombotic events, QT prolongation, hypertension, and left ventricular dysfunction. Furthermore, increased long-term survival rates among cancer patients treated with TKIs have brought attention to the significance of controlling long-term cardiovascular issues, which has accelerated the expansion of this market.
The Drug-Induced Cardiotoxicity market was dominated by North America region in 2025. The region's strong regulatory frameworks, sophisticated healthcare infrastructure, and increased knowledge of medication safety are all responsible. Significant investments in pharmaceutical and biotechnology research are particularly evident in the United States, which highlights the hunt for novel therapeutic approaches with low cardiotoxic consequences.
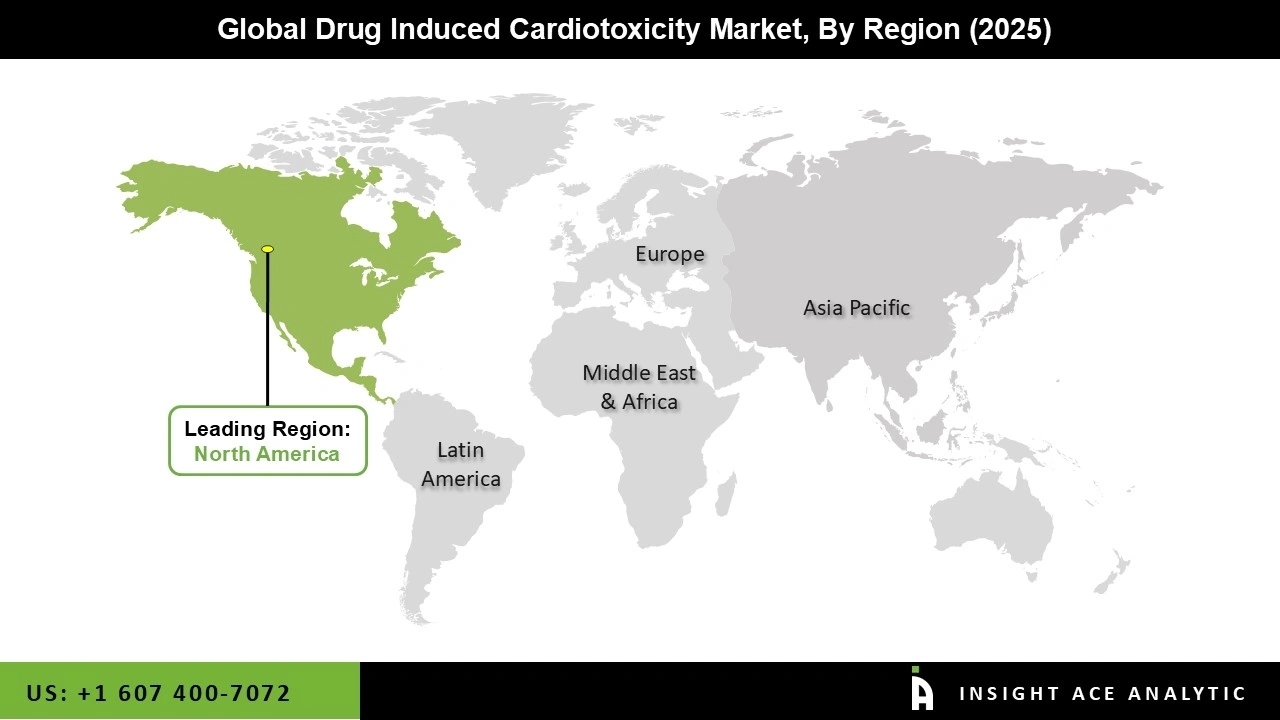
Additionally, the region's top pharmaceutical companies are actively funding research and development to lessen the cardiotoxic effects of drugs, which supports the market's growth. Furthermore, regional market growth is reinforced by the presence of top biotechnology and pharmaceutical businesses as well as continuous clinical research aimed at developing safer medicine formulations and cardioprotective drugs.
• September 2025: Tourmaline Bio, a clinical-stage biopharmaceutical startup, was fully acquired by Novartis, which now has it in its cardiovascular pipeline. After shareholders accepted a tender offer at $48 per share, the purchase, which was valued at about $1.4 billion in cash, was completed, and Tourmaline became an indirect wholly owned subsidiary of Novartis.
| Report Attribute | Specifications |
| Market size value in 2025 | USD 1.70 Bn |
| Revenue forecast in 2035 | USD 2.79 Bn |
| Growth Rate CAGR | CAGR of 5.2% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | Type, Drug Class, Detection, End-user, and By Region |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; France; Italy; Spain; South Korea; Southeast Asia |
| Competitive Landscape | Siemens Healthineers, Novartis AG, Pfizer Inc, GE Healthcare, Bristol Myers Squibb, Amgen Inc, and Others |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.