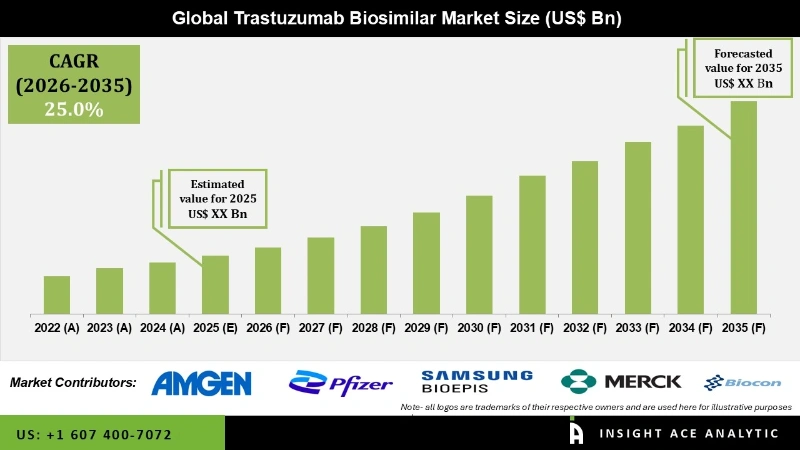
Trastuzumab Biosimilar Market is expected to grow at a 25.0 % CAGR during the forecast period for 2026 to 2035.
Trastuzumab Biosimilar Market Size, Share & Trends Analysis Report By Product (Ogivri, Herzuma, Ontruzant, Trazimera, Other Pipeline Products), By Indication, By Route of Administration, By Molecule Type, Region And Segment Forecasts, 2026 to 2035.
Trastuzumab is a recombinant DNA-derived humanized monoclonal antibody that binds to human epidermal growth factor receptor 2 (HER2). HER2 is over expressed in some types breast cancer cells hence, trastuzumab is mainly indicated for the treatment of HER2 positive breast cancer as well as it is also approved for the treatment of metastatic breast cancer and gastric cancer. It is marketed under the trade name of Herceptin by Roche. It is approved by U.S. Food and Drug Administration (FDA) in 1998 and by European Medicines Agency in 2000. Trastuzumab was Roche’s first targeted anticancer drug which accounts more than 90% of HER2 positive cancer market.
Trastuzumab is world’s second monoclonal antibody which has resulted in a better prognosis for breast cancer (HER2 positive) and improved survival rates. As a cost of Herceptin is so high therefore there is great demand for affordable medicines so that it can fulfil the need of low-income patients as well as expand its coverage. Biosimilar is defined as a type of biological products that are approved by respective regulatory bodies because of its clinical similarity to already approved biological products.
Patent of Herceptin expired in Europe in 2014, in Asia in 2017, and in the U.S. in 2019. Thus many of biosimilar of trastuzumab have entered into the market. For instance, in June 2019, FDA has approved Allergan and Amgen’s biosimilar of Herceptin, for the breast cancer. Additionally, in December 2019, Biocon Ltd. and Mylan N.V. has launched the Ogivri, a biosimilar to Herceptin (trastuzumab).
The patent expiry of the key trastuzumab biologics are expiring and several companies are entering into development of biosimilar for trastuzumab biosimilar. Introduction to biosimilar in the market, increase in incidence and prevalence rate of breast and gastric cancer, rising demand for targeted therapy, and price cuts have boosted the growth of trastuzumab biosimilar market. The price of trastuzumab biosimilar is predicted to be 80% of that of Herceptin thus making it more affordable and accessible in low and medium income developing countries. However, stringent regulatory process for the approval of trastuzumab biosimilar is major restrain for the growth of the trastuzumab biosimilar market.
The Global Trastuzumab Biosimilar market is segmented into Product, Indication, Route of administration, Molecule Type, Distribution Channel, and region. Based on product, ogivri, herzuma, ontruzant, trazimera, and other pipeline products. Based on indication, the global trastuzumab biosimilar market is segmented into adjuvant breast cancer, metastatic breast cancer, metastatic gastric cancer, and further indication.
In Case of the segment Route of administration, it comprises Subcutaneous, Intravenous, Parenteral, and Molecule Type. Whereas the molecule type segment includes Monoclonal antibodies, Peptide, Protein, Small molecule, and Product Type. Based on distribution channels the global market is segmented as hospital pharmacy, online pharmacy, and others direct distribution channels. The hospital pharmacy segment is expected to grow during the forecast period due to an increase in the number of hospital visits and hospital stays.
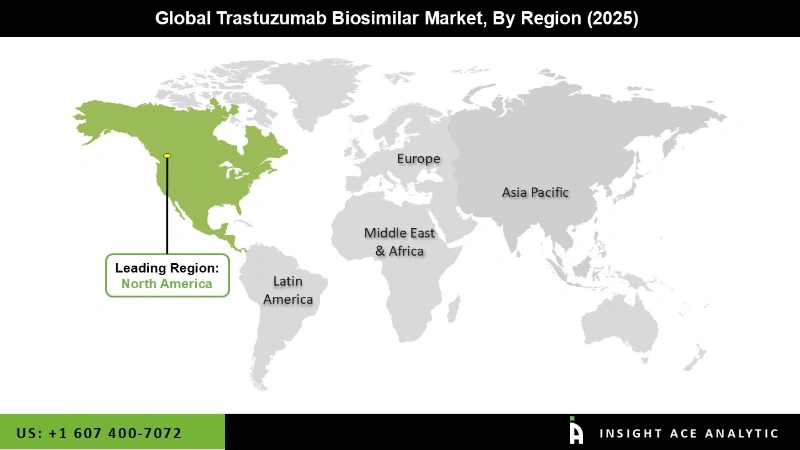
At the regional level, the Global Trastuzumab Biosimilar Market has been segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. In terms of revenue, Europe is a major contributor to the global trastuzumab biosimilar market. This is partly because of a trastuzumab patent has been expired in 2014, and in Nov 2017, Samsung Bioepis launched trastuzumab biosimilar in Europe. Followed by Europe, North America is estimated to rank second in the global market owing to patent expiry of Herceptin in 2019, and launch of a biosimilar for same are expected to propel the growth of the market in the region.
Additionally, strong clinical pipeline, increasing research and drug development activities propels market growth in the region. Asia Pacific is third promising revenue contributor which is expected to grow at a rapid pace in the upcoming year. Countries such as Japan, India, and China are major contributors to this market. Emerging and huge population base countries such as China and India offer tremendous market opportunities.
| Report Attribute | Specifications |
| Growth Rate CAGR | CAGR of 25.0 % from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Million and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Product, By Indication, By Route of Administration, By Molecule Type |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Amgen Inc., Pfizer Inc., Samsung Bioepis Co., Ltd., Merck & Co., Biocon Limited, Mylan Inc., BioXpress Therapeutics SA, Celltrion, EirGenix, Inc., Teva Pharmaceutical Industries Ltd., ALTEOGEN Inc., Apotex (Apobiologix), AryoGen Pharmed, BIOCAD, Prestige BioPharma (PBP), PlantForm, Outlook Therapeutics (Oncobiologics), Shanghai CP Guojian Pharmaceutical, Shanghai Henlius Biotech, and Stada Arzneimittel AG among others. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Trastuzumab Biosimilar Market By Product
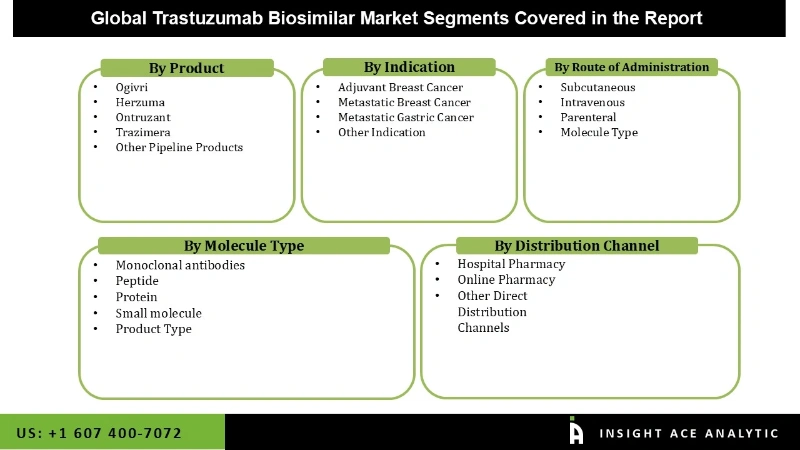
Global Trastuzumab Biosimilar Market By Indication
Global Trastuzumab Biosimilar Market By Route of Administration
Global Trastuzumab Biosimilar Market By Molecule Type
Global Trastuzumab Biosimilar Market By Distribution Channel
Global Trastuzumab Biosimilar Market By Region
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.