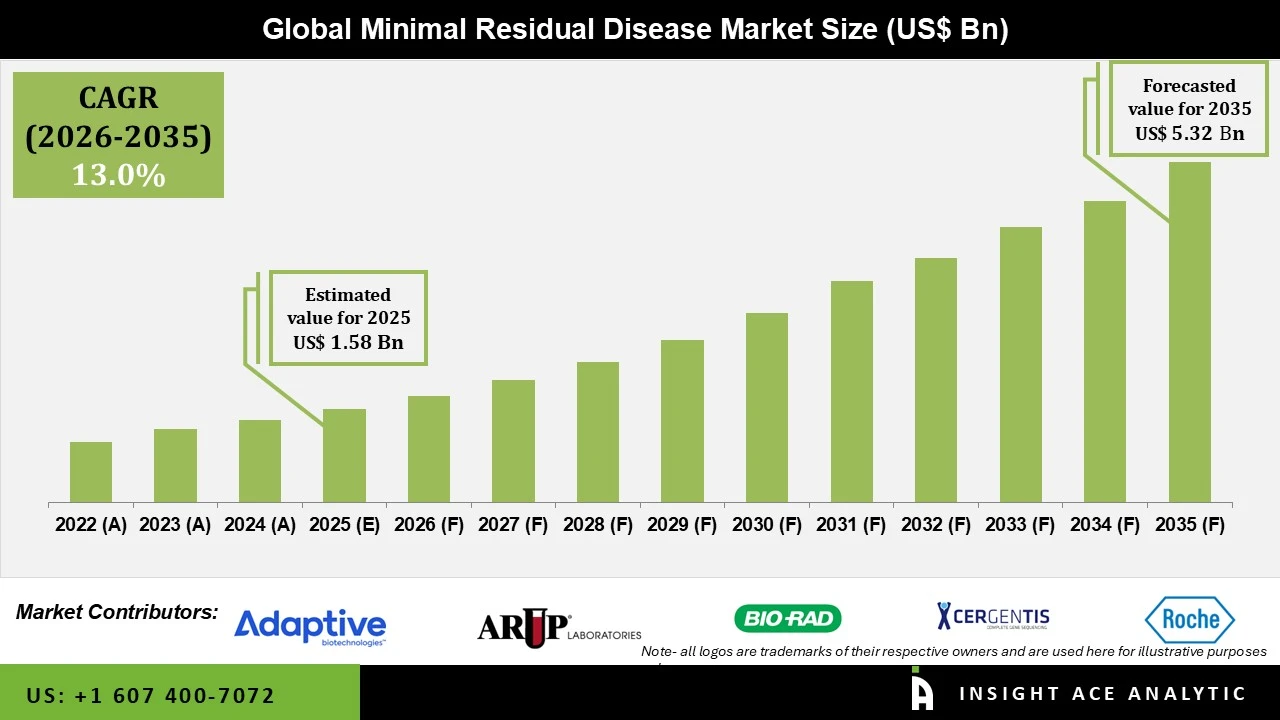
Minimal Residual Disease Market Size is valued at USD 1.58 Bn in 2025 and is predicted to reach USD 5.32 Bn by the year 2035 at a 13.0% CAGR during the forecast period for 2026 to 2035.
Minimal Residual Disease Market, Share & Trends Analysis Report, By Product (Assay Kits & Reagents, Instruments), By Technology (Polymerase Chain Reaction, Next-Generation Sequencing, Flow Cytometry, Other Technologies), By Application (Hematological Malignancies, Lymphoma, Solid Tumors, Multiple Myeloma, Other Applications), By End User, By Region, and Segment Forecasts, 2026 to 2035
Key Industry Insights & Findings from the Report:
The sophisticated nature of MRD detection methods, including next-generation sequencing (NGS), flow cytometry, and polymerase chain reaction (PCR), present challenges Minimal residual disease, also known as measurable residual disease or MRD, refers to the preclinical stages of diseases such as acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and multiple myeloma (MM). After treatment, the cancer cells that remain in the body are referred to as a minimal residual disease. These cancer cells are undetected by imaging or physical exams, but sophisticated laboratory tests can discover them. The minimal residual disease is an essential indicator of disease progression and therapy response. The growing importance of the test is expected to drive growth in the worldwide minimum residual disease market in the coming years.
The global incidence of cancer has increased dramatically, which is projected to play a vital role in expanding the global minimal residual illness market. Patients are expected to see more precision in treatment using novel technology. These factors are likely to have an impact on growth. Furthermore, favorable government and private-sector initiatives for developing and adopting NGS technologies, technological advancements in cloud computing and data integration, and the availability of a technologically advanced healthcare research framework are all stimulating market growth. Additionally, the improved regulatory and reimbursement landscape for NGS-based diagnostic tests and increased genome mapping initiatives will fuel market expansion throughout the forecast period.
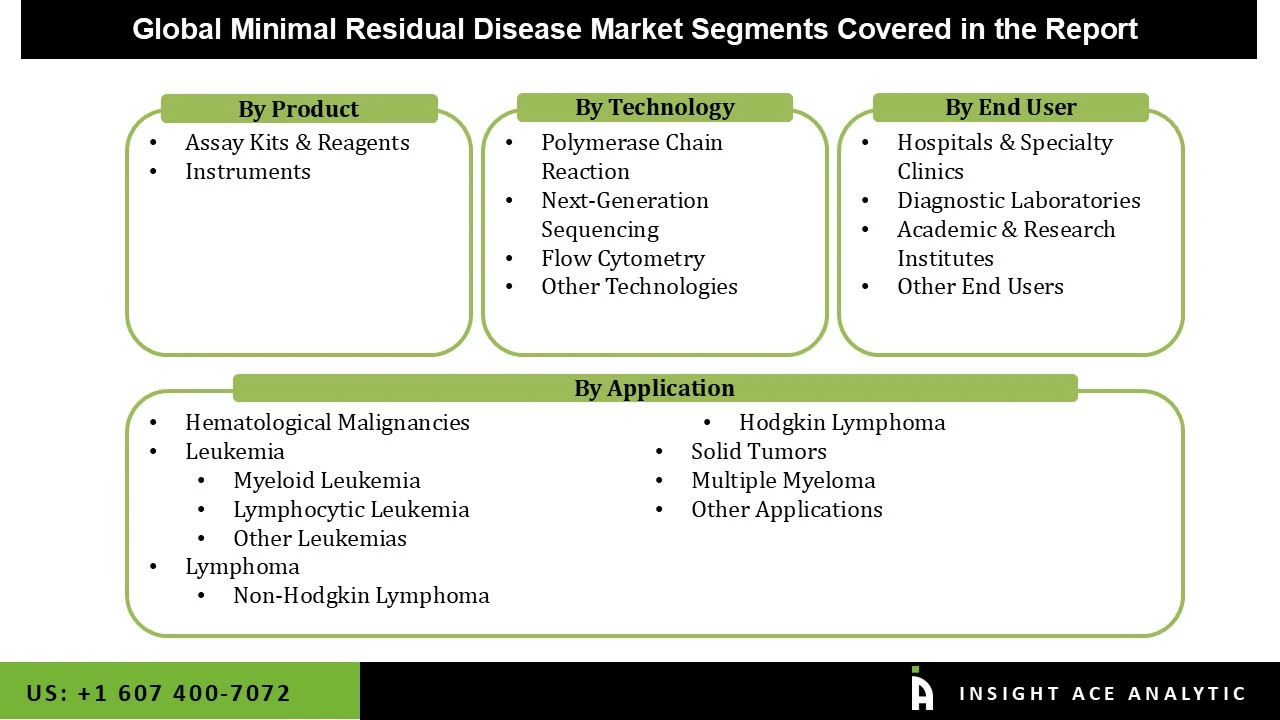
The minimal residual disease market is segmented on the product, technology, application and end user. Based on product, the minimal residual disease market is divided into assay kits & reagents, instruments. Based on the technology, the minimal residual disease market is divided into polymerase chain reaction, next-generation sequencing, flow cytometry, and other technologies. Based on the Application, the minimal residual disease market is segmented into hematological malignancies, lymphoma, solid tumors, multiple myeloma, and other applications. Based on the end user, the minimal residual disease market is segmented into hospitals & specialty clinics, diagnostic laboratories, academic & research institutes, and other end users.
Based on product, the minimal residual disease market is divided into assay kits & reagents, instruments. Among these, The Assay Kits & Reagents segment leads the MRD detection market due to its frequent and recurring use, as each test requires fresh reagents and assay kits. This segment benefits from the growing demand for repeat testing to monitor MRD levels during and after treatment, making it essential for effective disease management. Additionally, the expanding use of MRD detection in personalized medicine and clinical trials further fuels the demand for these consumables. In contrast, instruments such as flow cytometers and PCR machines represent a smaller yet stable market share, primarily driven by infrastructure investment rather than test volume.
Based on the technology, the minimal residual disease market is divided into polymerase chain reaction, next-generation sequencing, flow cytometry, and other technologies. Among these, the Polymerase Chain Reaction (PCR) segment has traditionally dominated the MRD detection market due to its widespread use, high specificity, and cost-effectiveness in routine clinical monitoring. However, Next-Generation Sequencing (NGS) is emerging as the fastest-growing segment, driven by its ultra-high sensitivity and ability to detect a broad spectrum of genetic alterations, making it increasingly favored in clinical trials and personalized treatment approaches. Flow cytometry also holds a significant share, especially in acute leukemias, offering rapid and relatively affordable results, though with lower sensitivity.
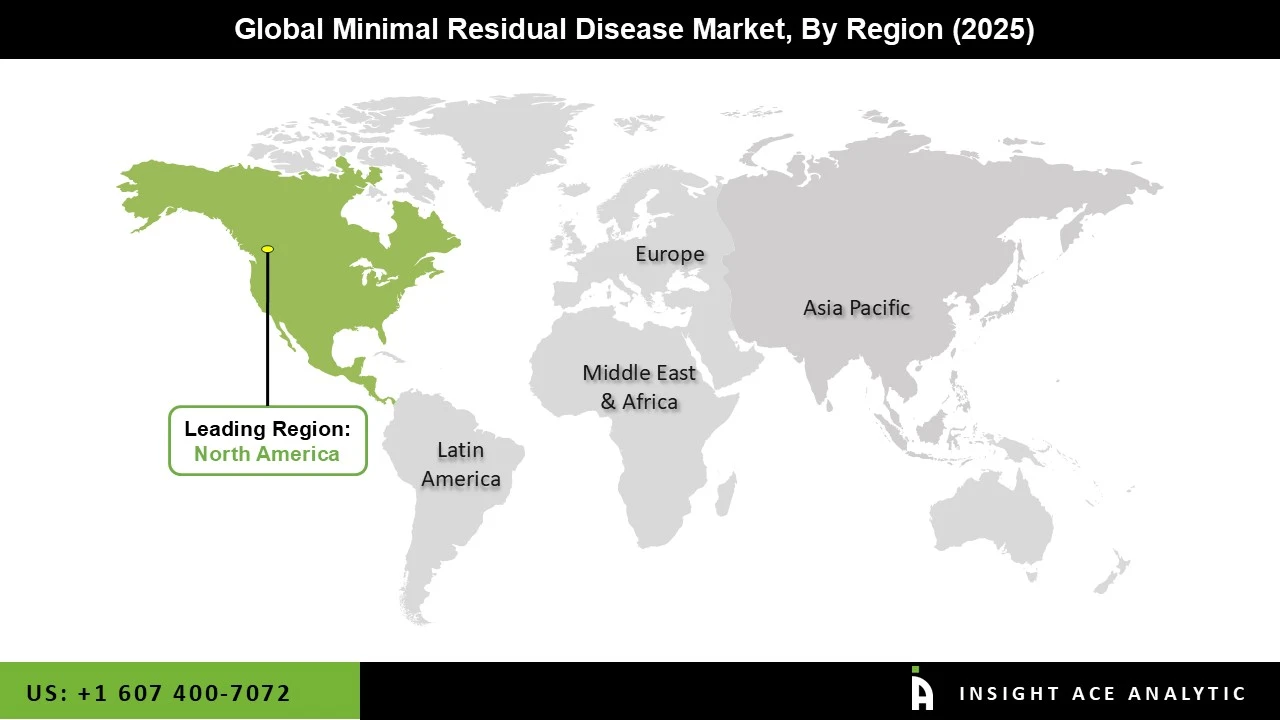
The North American minimal residual disease market is expected to register the highest market share in revenue shortly. The rising prevalence of blood malignancies and the increased investment in MRD research by government and private groups have grown healthcare affordability in the United States and the rise in knowledge and awareness among individuals in this region. In addition, Asia Pacific is projected to grow rapidly in the global minimal residual disease market due to government initiatives and large-scale genome sequencing studies funding to develop targeted treatments to contribute to market growth. Furthermore, the existence of key organizations and industries and the quick spread of paternity testing, genealogy, and personal health awareness drive market growth in this area over the forecast period.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 1.58 Bn |
| Revenue Forecast In 2035 | USD 5.32 Bn |
| Growth Rate CAGR | CAGR of 13.0% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn,and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | Product, Technology, Application |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ; France; Italy; Spain; South Korea; South East Asia; |
| Competitive Landscape | F. Hoffmann-La Roche Ag, Laboratory Corporation of America Holdings, Guardant Health, Inc, Sysmex Corporation, NEOGENOMICS Laboratories, MOLECULARMD, Adaptive Biotechnologies, ARCHERDX, Bio-Rad Laboratories, Inc, Natera, Inc, OPKO Health, Inc, GENETRON Health, Quest Diagnostics, Inc, ASURAGEN, Inc, INVIVOSCRIBE, Inc, Arup Laboratories Inc, Mission Bio, Inc, ERGENTIS B.V |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Minimal Residual Disease Market By Product
Minimal Residual Disease Market By Technology
Minimal Residual Disease Market By Application
Minimal Residual Disease Market By End User
By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.