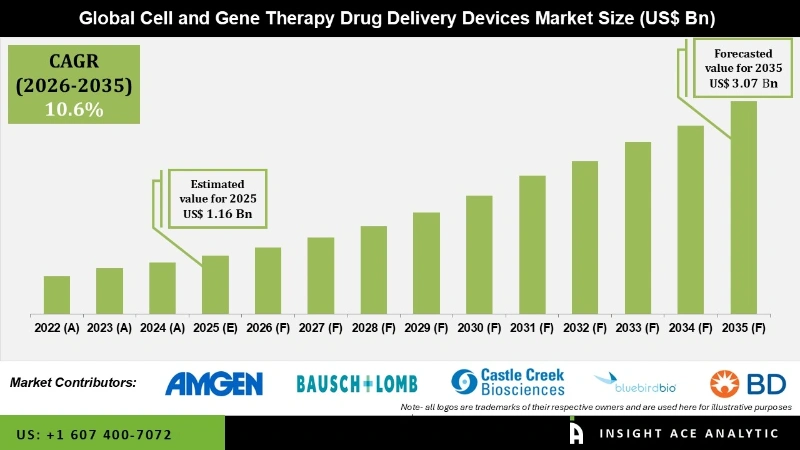
Global Cell and Gene Therapy Drug Delivery Devices Market Size is valued at USD 1.16 Bn in 2025 and is predicted ato reach USD 3.07 Bn by the year 2035 at a 10.6% CAGR during the forecast period for 2026 to 2035.
Cell and Gene Therapy Drug Delivery Devices Market Size, Share & Trends Analysis Report By Products (Subretinal Injection Cannula, Extension Tube, Intravenous Catheter, Sterile Insulin Syringe, Pre-Filled Syringe, and Infusion Bags), Commercialized Drugs, Region, And Segment Forecasts, 2026 to 2035.
Key Industry Insights & Findings from the Report:
The demand for cell and gene therapy drug delivery devices market is enhancing as there is an increase in the number of patients suffering from cancer, diabetes, and other chronic diseases. Therefore, there is a high need of developing affordable and cost-effective cell and gene therapies. Cell and gene therapy is a clinical procedure of modifying genetic material for the treatment of various diseases. Drug Delivery devices have the capacity to perform multiple functions with the highest degree of smartness. Cell and Gene Therapy Drug Delivery Devices are beneficial in the treatment of respiratory diseases, diabetes, and other cardiovascular disorders.
Spreading awareness regarding the benefits of cell and gene therapy drug delivery devices is expected to propel the product demand. Furthermore, fast adoption of advanced technologies, an invention of new devices according to patient’s requirements, increasing government initiatives for research and development of new therapies and devices, high prevalence of chronic diseases, rising number of clinical trials, and creation of smartphone applications will also help to upscale this market. However, the high cost of manufacturing systems, lack of standard therapy protocols, complex procedures, and shortage of skilled professionals are limiting the growth of this market.
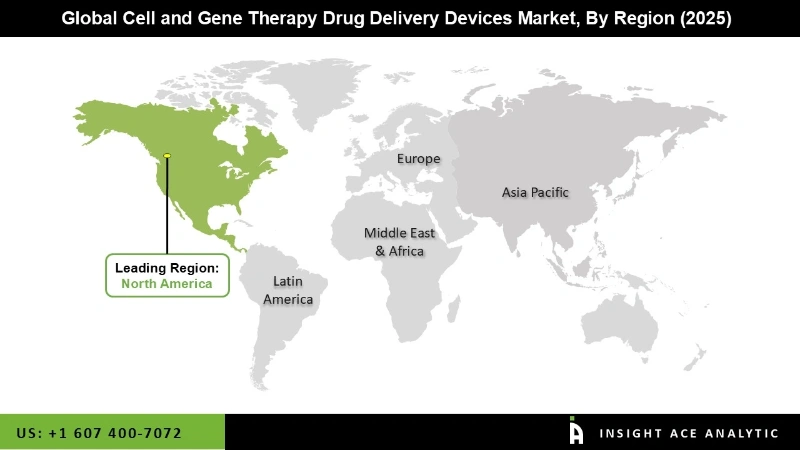
The cell and gene therapy drug delivery devices market can be segmented on the basis of products, Commercialized Drugs, and Regions. On the basis of products, the market can be sub-divided into Subretinal Injection Cannula, Extension Tube, Intravenous Catheter, Sterile Insulin Syringe, Pre-Filled Syringe, and Infusion Bags. On the basis of Commercialized Drugs, the market is sub-segmented into Luxturna, Kymriah, Provenge, Zolgensma, Yescarta, and Strimvelis. Commercialized Drugs segment is expected to dominate this market, as these drugs are modified and prepared according to patient’s requirements. In terms of geography, the market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. Out of these regions, North America is expected to dominate the market over the forecast period (2019-2031).
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 1.16 Bn |
| Revenue Forecast In 2035 | USD 3.07 Bn |
| Growth Rate CAGR | CAGR of 10.6% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Thousands and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Products, By Commercialized Drugs |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
| Competitive Landscape | Bausch & Lomb Incorporated., Amgen Inc., Becton, Dickinson and Company, Castle Creek Biosciences, Inc. (Fibrocell Science, Inc.), Dendreon Pharmaceuticals LLC., Bluebird bio, Inc., Helixmith Co., Ltd (ViroMed Co., Ltd), Human Stem Cells Institute, Kite Pharma, Inc., Novartis AG, Orchard Therapeutics plc., Pfizer, Inc., Renova Therapeutics, Spark Therapeutics, Inc., Kolon TissueGene, Inc., Vericel Corporation, uniQure N.V., and Others |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Cell and Gene Therapy Drug Delivery Devices Market by Products
Global Cell and Gene Therapy Drug Delivery Devices Market by Commercialized Drugs
Global Cell and Gene Therapy Drug Delivery Devices Market by Region
Europe -
North America -
Asia Pacific -
Latin America -
Middle East & Africa -
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.