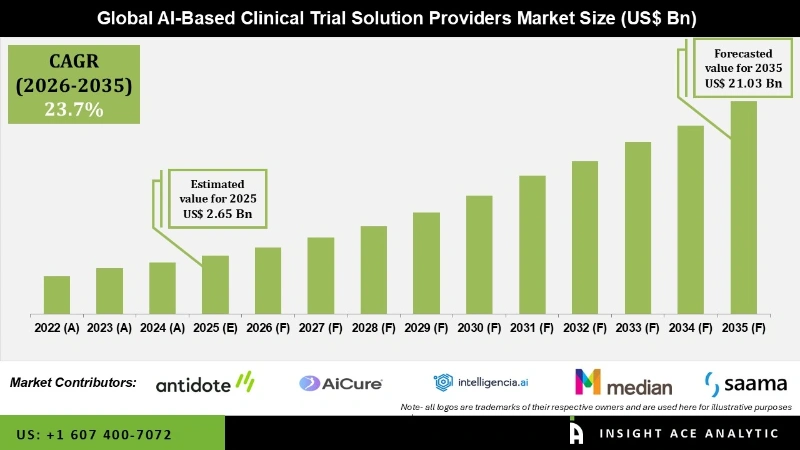
Global AI-Based Clinical Trial Solution Providers Market Size is valued at USD 2.65 Billion in 2025 and is predicted to reach USD 21.03 Billion by the year 2035 at a 23.7% CAGR during the forecast period for 2026 to 2035.
AI-Based Clinical Trial Solution Providers Market Size, Share & Trends Analysis Report By Target Therapeutics (Cardiovascular Disorders, CNS Disorders, Infectious Disorders, Metabolic Disorders, Oncological Disorders, and Other Disorders), By Trial Phase, By End Users, Region And Segment Forecasts, 2026 to 2035.
Artificial Intelligence is a set of smart technologies that develop and learn from data and perform tasks based on previous experience. The process, clinical trials are crucial that enable both innovators and regulators to assess the efficacy of a candidate drug. The use of AI in clinical trial saves time and cost efficiencies by providing faster insights to form the decision. AI helps investigators to answer some questions, including how to construct study design, site identification & patient recruitment for clinical research, and digitize adverse drug reaction (ADR) documents in pharmacovigilance. Successfully developing a novel therapeutic intervention required around 10 years of time and cost around USD 2.5 billion. However, the use of technology like artificial intelligence in a clinical trial help to save a huge amount of money and time.
Increasing the number of strategic alliances in deployment of Artificial Intelligence (AI) in clinical trials aims to reduce time and expenditure during clinical developmental phases is expected to create lucrative growth in market in the near future. Additionally, regulators worldwide have released guidelines that encourage biopharma companies to use real-world evidence strategies. For instance, US FDA has passed the 21st Century Cures Act, in 2016, that was designed to help bring new innovations and advances to patients more efficiently and faster.
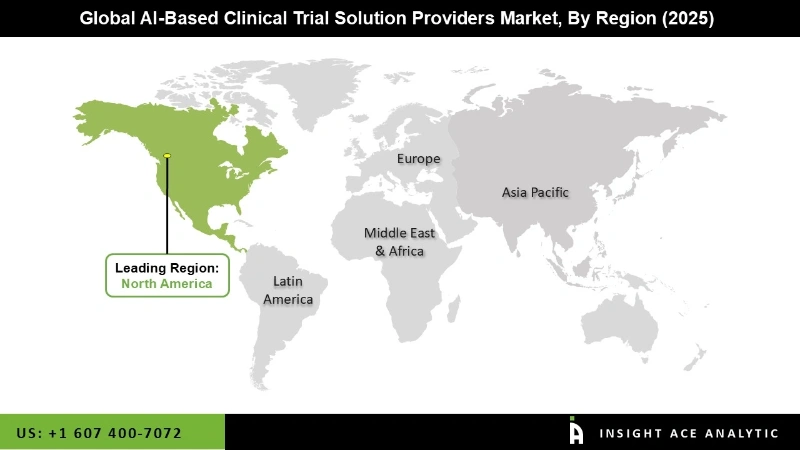
Global AI-Based Clinical Trial Solution Provider market is segmented on the basis of target therapeutic area, trial phase, end users and region. Based on the therapeutic area, the market is divided into cardiovascular disorders, cns disorders, infectious disorders, metabolic disorders, oncological disorders and other disorders. Based on the trial phase, the market is divided into early phase 1, phase 1, phase 2, phase 3 and phase 4. Based on the end users, the market is divided into pharmaceutical companies, academia and other users. Based on region, the market is studied across North America, Asia-Pacific, Europe, and LAMEA. Among that Europe held the largest share of the market, followed by America and Asia Pacific. On the other hand, North America is expected to dominate the market during the analysis of forecast period.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 2.65 Billion |
| Revenue Forecast In 2035 | USD 21.03 Billion |
| Growth Rate CAGR | CAGR of 23.7% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$Mn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Target Therapeutics, By Trial Phase, By End Users |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
| Competitive Landscape | Antidote Technologies, Inc., AiCure, LLC, Deep 6 AI, Deep Lens Inc., Innoplexus, Intelligencia.ai, MEDIAN Technologies, Mendel.ai, Phesi, Saama Technologies, Unlearn.AI, Inc. and Trials.ai, and others |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) by Target Therapeutics
Global AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) Based on Trial Phase
Global AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) Based on end users
Global AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) Based on Region
Europe AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) by Country
North America AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) by Country
Asia Pacific AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) by Country
Latin America AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) by Country
Middle East & Africa AI-Based Clinical Trial Solution Provider Market Revenue (US$ Mn) by Country
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.