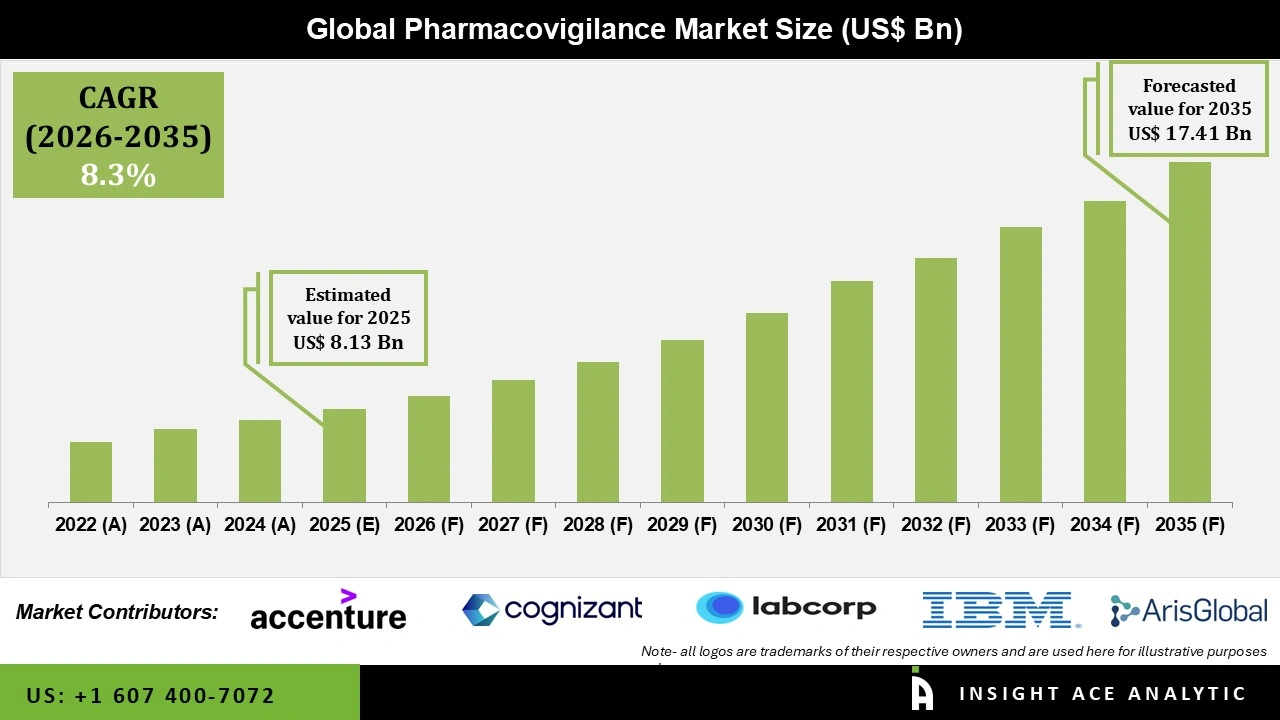
Global Pharmacovigilance Market Size is valued at USD 8.13 Bn in 2025 and is predicted to reach USD 17.41 Bn by the year 2035 at a 8.3% CAGR during the forecast period for 2026 to 2035.
Pharmacovigilance Market Size, Share & Trends Analysis Report By Service Provider (In-House And Contract Outsourcing), Product Life Cycle (Pre-Clinical, Phase I, Phase II, Phase III And Phase IV), By Type, By Process Flow, By Therapeutic Area, And By End-Use, By Region, and Segment Forecasts, 2026 to 2035
Key Industry Insights & Findings from the Report:
The pharmacovigilance market refers to the global industry of monitoring and assessing the safety and efficacy of drugs and medical products after being approved. The market includes various services and solutions, such as adverse event reporting, signal detection, risk management, regulatory compliance, and post-market surveillance.
In recent years, the pharmacovigilance market has experienced substantial growth due to the rising prevalence of chronic diseases, rising demand for personalized medicines, and growing regulatory requirements for drug safety. Advancements also drive the market in healthcare technologies, such as artificial intelligence, big data analytics, and blockchain, which have enhanced the efficiency and accuracy of pharmacovigilance processes.
Despite the increasing focus on drug safety, there is still a lack of awareness about pharmacovigilance among healthcare professionals and the general public. This could result in underreporting adverse drug reactions and delays in detecting safety signals.
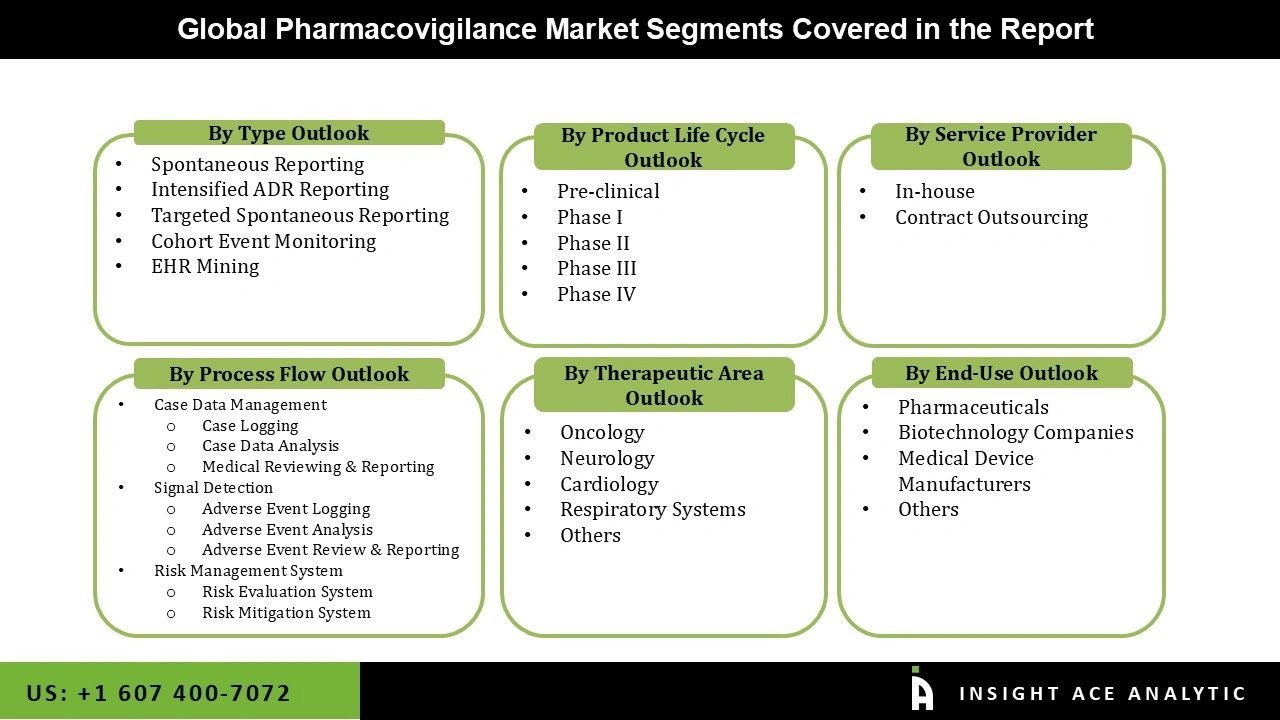
The pharmacovigilance market is segmented based on the service provider, product life cycle, type, process flow, therapeutic area, and end-use. Based on the service provider, the market is segmented as in-house and contract outsourcing. Based on the product life cycle, the market is segmented into pre-clinical, phase I, phase II, phase III and phase IV. Based on type, the market is segmented into spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring and EHR mining.
Based on process flow, the market is segmented into case data management, case logging, case data analysis, medical reviewing & reporting signal detection, adverse event logging, adverse event analysis, adverse event review & reporting, risk management system, risk evaluation system and risk mitigation system. Based on therapeutic areas, the market is segmented into oncology, neurology, cardiology, and respiratory systems. Based on end-use, the market is segmented into pharmaceuticals, biotechnology companies, medical device manufacturers and others.
Contract outsourcing is one of the dominant segments in the pharmacovigilance market, and it is anticipated to continue dominating the demand in the coming years. Contract outsourcing involves outsourcing pharmacovigilance services to third-party service providers, such as contract research organizations (CROs) and business process outsourcing (BPO) companies. Factors such as cost-effectiveness, improved efficiency, and access to specialized expertise drive the contract outsourcing segment. Outsourcing pharmacovigilance services enables pharmaceutical companies to focus on their core competencies while leveraging the expertise of specialized service providers to manage the complexities of drug safety.
Moreover, outsourcing pharmacovigilance services helps companies to reduce costs associated with hiring and training personnel, managing infrastructure, and maintaining compliance with regulatory requirements. This has led to an increasing trend of outsourcing pharmacovigilance services to third-party service providers, especially among small and mid-sized pharmaceutical companies.
Spontaneous reporting is an essential component of pharmacovigilance, and it is expected to continue dominating the pharmacovigilance market in the coming years. The spontaneous reporting segment is driven by various factors, such as the widespread use of drugs and medical products, increasing awareness about drug safety, and the need for early detection and management of ADRs. Spontaneous reporting plays a crucial role in identifying and assessing the safety of drugs and medical products after being approved.
The Asia-Pacific pharmacovigilance market is expected to register the highest market share in revenue shortly. The Asia-Pacific region is anticipated to dominate the pharmacovigilance market in the coming years. The region is expected to exhibit the highest growth rate due to various factors, such as the increasing demand for pharmaceuticals, rising prevalence of chronic diseases, growing regulatory requirements, and rising awareness about drug safety and adverse effects.
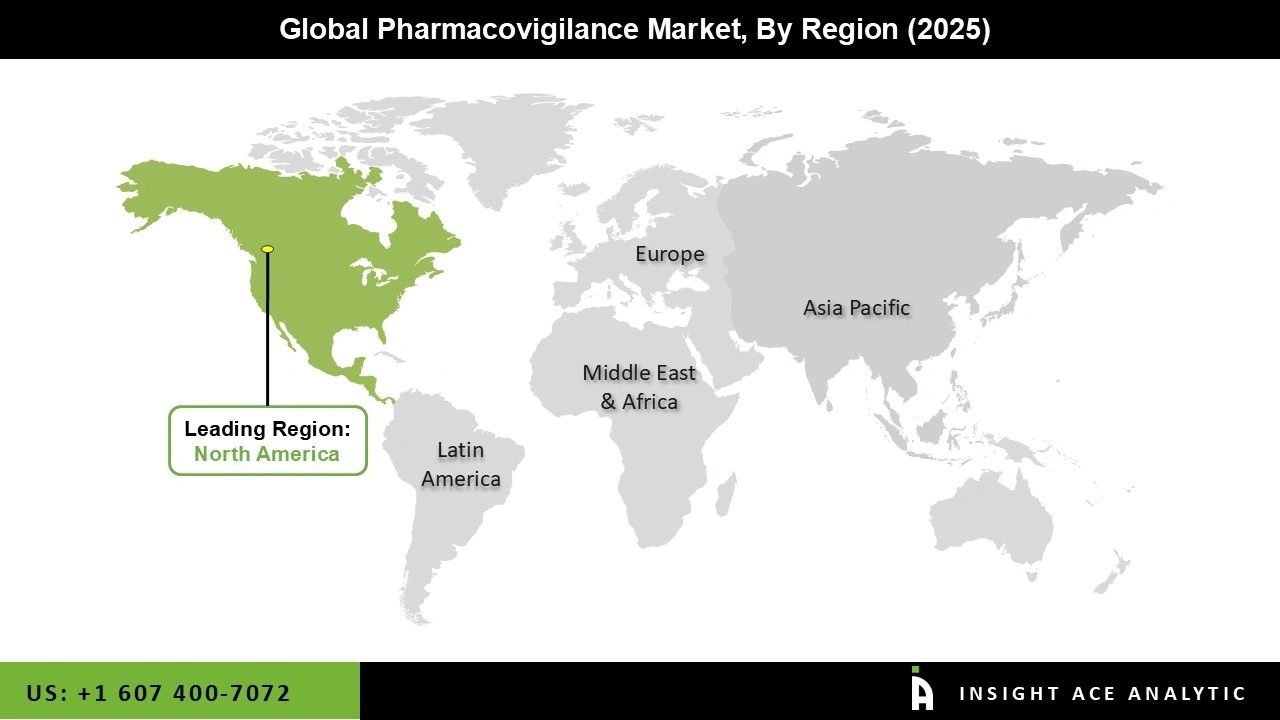
Countries like China and India have been experiencing significant growth in their pharmaceutical industries, leading to increased demand for pharmacovigilance services. Moreover, the growing emphasis on patient safety and the need for regulatory compliance has further boosted the demand for pharmacovigilance solutions in the region. Besides, North America is currently the largest market for pharmacovigilance and is anticipated to continue dominating the market in the coming years. Various factors, including many pharmaceutical companies, advanced healthcare infrastructure, and increasing demand for personalized medicine, drive the North American pharmacovigilance market. The region has stringent regulatory requirements for drug safety, leading to the establishment of a well-developed pharmacovigilance infrastructure.
| Report Attribute | Specifications |
| Market size value in 2025 | USD 8.13 Bn |
| Revenue forecast in 2035 | USD 17.41 Bn |
| Growth rate CAGR | CAGR of 8.3% from 2026 to 2035 |
| Quantitative units | Representation of revenue in US$ Bn, and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026 to 2035 |
| Report coverage | The forecast of revenue, the position of the company, the competitive market statistics, growth prospects, and trends |
| Segments covered | Service Provider, Product Life Cycle, Type, Process Flow, Therapeutic Area, And End-Use |
| Regional scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; Southeast Asia |
| Competitive Landscape | Accenture; LinicalAccelovance; Cognizant; Laboratory Corporation of America Holdings; IBM Corporation; ArisGlobal; ICON plc.; Capgemini; ITClinical; FMD K&L; IQVIA; TAKE Solutions Ltd.; PAREXEL International Corporation; BioClinica Inc.; Wipro Ltd.; and United BioSource Corporation |
| Customization scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and available payment methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.