Global Advanced Drug Delivery CDMO Market Size is predicted to show a 5.00% CAGR during the forecast period for 2026 to 2035.
Advanced Drug Delivery CDMO Market Size, Share & Trends Analysis Report By Service Type (Formulation Development, Process Development and Scale-Up, Analytical and Testing Services, Manufacturing Services, Packaging and, Device Development, Regulatory Support), By Technology (Targeted Drug Delivery Systems, Controlled/Sustained-Release Technologies, Injectable Delivery Platforms, Pulmonary & Nasal Delivery Systems, Transdermal & Microneedle Delivery, Mucosal Delivery Methods, Implantable Delivery Devices, 3D-Printed Drug Delivery), By Dosage Form, By Application, By End-User By Region, And By Segment Forecasts, 2026 to 2035.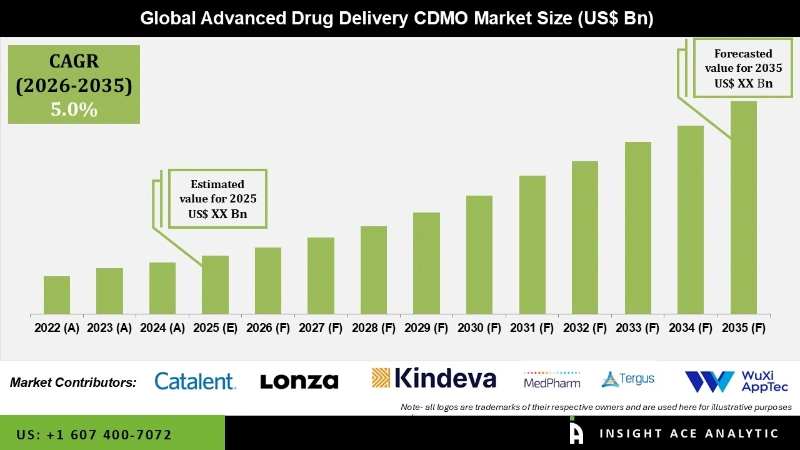
The pharmaceutical sector greatly benefits from a Contract Development and Manufacturing Organisation (CDMO) specialising in cutting-edge drug delivery technologies. These CDMOs offer services for creating, formulating, and developing drug delivery systems beyond conventional oral tablets or capsules. Modern medication delivery systems have arisen as a quick and precise way to get the drug to the right place. These systems have several advantages over conventional systems, including the focused and regulated delivery of novel molecules to target areas, consistent drug absorption, reduced frequency of dosing, decreased production of hazardous metabolites, and lower variability in systemic drug concentrations.
The need for advanced drug delivery CDMOs has increased as a result of improved drug performance and superior results brought about by advanced drug delivery systems. However, the Advanced Drug Delivery Contract Development and Manufacturing Organisation (CDMO) market was significantly impacted by the COVID-19 pandemic, which had a wide-ranging effect on the sector. The pandemic affected the availability of raw materials and parts needed for medicine delivery systems by upsetting global supply chains. For several CDMOs, this resulted in manufacturing difficulties and delivery delays.
Some Major Key Players in the Advanced Drug Delivery CDMO Market are:
The advanced drug delivery CDMO market is segmented on the basis of services which is segmented as service type, technology, dosage form, application and end-user. Based on the service type, the market segmented into formulation development, process development and scale-up, analytical and testing services, manufacturing services, packaging and, device development, regulatory support. Based on the technology, the market segmented into targeted drug delivery systems, controlled/sustained-release technologies, injectable delivery platforms, pulmonary & nasal delivery systems, transdermal & microneedle delivery, mucosal delivery methods, implantable delivery devices, 3d-printed drug delivery. Based on the dosage form, the market segmented into injectables, oral solids, topical & transdermal, implantables & ocular. Based on the application, the market segmented into oncology, infectious diseases, neurological disorders, cardiovascular diseases, other. Based on the end-user, the market segmented into clinical, commercial.
The analytical and testing services category is expected to hold a major share in the global Advanced Drug Delivery CDMO Market in 2025. Advanced drug delivery technologies such as liposomes, nanoparticles, implants, and long-acting injectables require sophisticated testing to ensure their performance, safety, and stability. These systems often exhibit complex release profiles, encapsulation efficiencies, and bioavailability parameters that must be validated using high-precision analytical methods. Analytical services are essential at every stage of the drug development lifecycle, from preformulation and clinical trials to regulatory submission and post-marketing surveillance. As a result, the Analytical and Testing Services segment holds the largest share in the advanced drug delivery CDMO market, as it plays a critical role in ensuring the quality, safety, regulatory compliance, and functional performance of increasingly complex drug delivery systems.
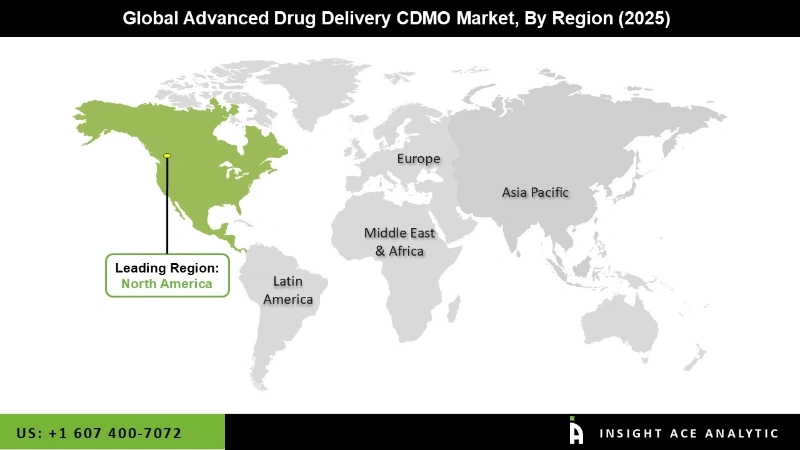
The North America Advanced Drug Delivery CDMO Market is expected to record the maximum market share in terms of revenue in the near future. The region's long-standing CDMO presence distinguishes the market in North America. The region is heavily invested in researching and developing biological medications, gene treatments, and other specialized pharmaceuticals. These complex compounds frequently necessitate specialized drug delivery devices, which drives demand for CDMO services. Along with this element, expanding partnerships between pharmaceutical firms and CDMOs providing a wide range of services are to blame for the region's leading market share globally. Due to the region's highly qualified workforce and low manufacturing and R&D costs, the Asia Pacific market is estimated to grow significantly over the course of the forecast period.
Recent Developments:
| Report Attribute | Specifications |
| Growth Rate CAGR | CAGR of 5.00% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Mn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Service Type, Technology, Dosage Form, Application, End-User |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; South East Asia |
| Competitive Landscape | Catalent, Inc., Lonza Group AG, Kindeva Drug Delivery, MedPharm (merged with Tergus Pharma), WuXi AppTec, Hovione, Piramal Pharma Solutions, CordenPharma International, Divi’s Laboratories, Lifecore Biomedical, Recipharm AB, Syngene International, Thermo Fisher Scientific (Patheon), Aenova Group, Cellipoint Bioservices, Evonik Health Care, SHL Medical, Lubrizol Life Science, Acino International AG, Pharmathen, Phillips-Medisize (A Molex Company), Others |
| Customization Scope | Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Segmentation of Advanced Drug Delivery CDMO Market -
Advanced Drug Delivery CDMO Market, By Service Type
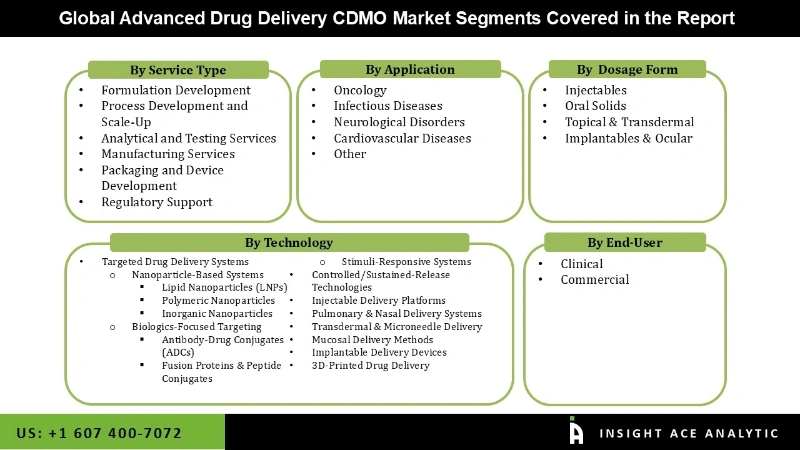
Advanced Drug Delivery CDMO Market, By Technology
Advanced Drug Delivery CDMO Market, By Dosage Form
Advanced Drug Delivery CDMO Market, By Application
Advanced Drug Delivery CDMO Market, By End-User
Advanced Drug Delivery CDMO Market, By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.