Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global Radiotherapy-Induced Nausea and Vomiting Treatment Market Snapshot
Chapter 4. Global Radiotherapy-Induced Nausea and Vomiting Treatment Market Variables, Trends & Scope
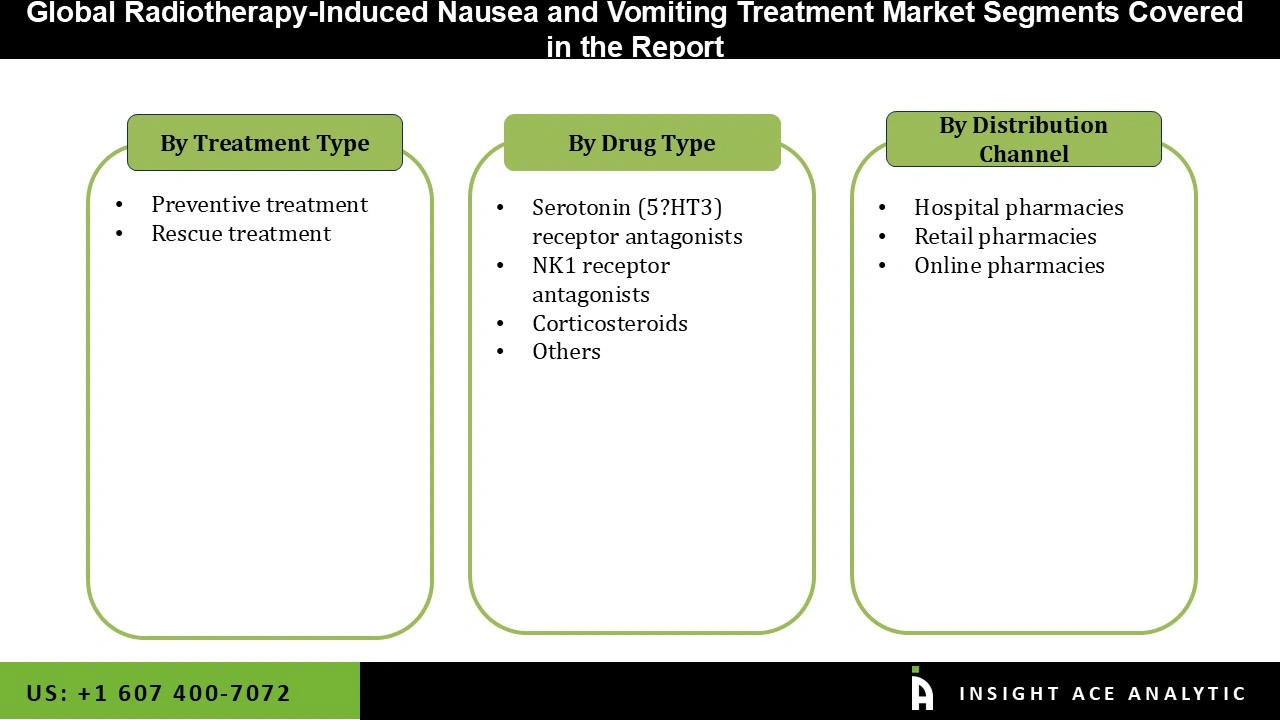
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Porter’s Five Forces Analysis
4.7. Incremental Opportunity Analysis (US$ Mn), 2026–2035
4.8. Global Radiotherapy-Induced Nausea and Vomiting Treatment Market Penetration & Growth Prospect Mapping (US$ Mn), 2025–2035
4.9. Competitive Landscape & Market Share Analysis, By Key Player (2025)
4.10. Use/Impact of AI on Antiemetic Therapy Development and Patient Management Trends
Chapter 5. Radiotherapy-Induced Nausea and Vomiting Treatment Market Segmentation 1: By Drug Type, Estimates & Trend Analysis
5.1. Market Share by Drug Type, 2025 & 2035
5.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2022–2035 for the following Drug Type:
5.2.1. Corticosteroids
5.2.2. Serotonin (5-HT3) Receptor Antagonists
5.2.3. NK1 Receptor Antagonists
5.2.4. Others
Chapter 6. Radiotherapy-Induced Nausea and Vomiting Treatment Market Segmentation 2: By Treatment Type, Estimates & Trend Analysis
6.1. Market Share by Treatment Type, 2025 & 2035
6.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2022–2035 for the following Treatment Type:
6.2.1. Preventive Treatment
6.2.2. Rescue Treatment
Chapter 7. Radiotherapy-Induced Nausea and Vomiting Treatment Market Segmentation 3: By Distribution Channel, Estimates & Trend Analysis
7.1. Market Share by Distribution Channel, 2025 & 2035
7.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2022–2035 for the following Distribution Channel:
7.2.1. Hospital Pharmacies
7.2.2. Online Pharmacies
7.2.3. Retail Pharmacies
Chapter 8. Radiotherapy-Induced Nausea and Vomiting Treatment Market Segmentation 4: Regional Estimates & Trend Analysis
8.1. Global Radiotherapy-Induced Nausea and Vomiting Treatment Market Regional Snapshot, 2025 & 2035
8.2. North America
8.2.1. North America Radiotherapy-Induced Nausea and Vomiting Treatment Market Revenue (US$ Mn) Estimates and Forecasts by Country, 2022–2035
8.2.1.1. United States
8.2.1.2. Canada
8.2.2. North America Market Revenue Estimates by Drug Type, 2022–2035
8.2.3. North America Market Revenue Estimates by Treatment Type, 2022–2035
8.2.4. North America Market Revenue Estimates by Distribution Channel, 2022–2035
8.2.5. North America Market Analysis
8.3. Europe
8.3.1. Europe Radiotherapy-Induced Nausea and Vomiting Treatment Market Revenue (US$ Mn) Estimates and Forecasts by Country, 2022–2035
8.3.1.1. Germany
8.3.1.2. United Kingdom
8.3.1.3. France
8.3.1.4. Italy
8.3.1.5. Spain
8.3.1.6. Rest of Europe
8.3.2. Europe Market Revenue Estimates by Drug Type, 2022–2035
8.3.3. Europe Market Revenue Estimates by Treatment Type, 2022–2035
8.3.4. Europe Market Revenue Estimates by Distribution Channel, 2022–2035
8.3.5. Europe Market Analysis
8.4. Asia Pacific
8.4.1. Asia Pacific Radiotherapy-Induced Nausea and Vomiting Treatment Market Revenue (US$ Mn) Estimates and Forecasts by Country, 2022–2035
8.4.1.1. China
8.4.1.2. Japan
8.4.1.3. India
8.4.1.4. South Korea
8.4.1.5. South East Asia
8.4.1.6. Rest of Asia Pacific
8.4.2. Asia Pacific Market Revenue Estimates by Drug Type, 2022–2035
8.4.3. Asia Pacific Market Revenue Estimates by Treatment Type, 2022–2035
8.4.4. Asia Pacific Market Revenue Estimates by Distribution Channel, 2022–2035
8.4.5. Asia Pacific Market Analysis
8.5. Latin America
8.5.1. Latin America Radiotherapy-Induced Nausea and Vomiting Treatment Market Revenue (US$ Mn) Estimates and Forecasts by Country, 2022–2035
8.5.1.1. Brazil
8.5.1.2. Argentina
8.5.1.3. Mexico
8.5.1.4. Rest of Latin America
8.5.2. Latin America Market Revenue Estimates by Treatment Type, 2022–2035
8.5.3. Latin America Market Revenue Estimates by Distribution Channel, 2022–2035
8.5.4. Latin America Market Analysis
8.6. Middle East & Africa
8.6.1. Middle East & Africa Radiotherapy-Induced Nausea and Vomiting Treatment Market Revenue (US$ Mn) Estimates and Forecasts by Country, 2022–2035
8.6.1.1. GCC Countries
8.6.1.2. South Africa
8.6.1.3. Rest of Middle East & Africa
8.6.2. Middle East & Africa Market Revenue Estimates by Treatment Type, 2022–2035
8.6.3. Middle East & Africa Market Revenue Estimates by Distribution Channel, 2022–2035
8.6.4. Middle East & Africa Market Analysis
Chapter 9. Competitive Landscape
9.1. Major Mergers, Acquisitions & Strategic Alliances
9.2. Company Profiles
9.2.1. Novartis AG
9.2.2. Pfizer Inc.
9.2.3. Sanofi S.A.
9.2.4. Teva Pharmaceutical Industries Ltd.
9.2.5. GlaxoSmithKline plc
9.2.6. Unimed Pharmaceuticals (AbbVie)
9.2.7. Merck & Co., Inc.
9.2.8. Cipla Ltd.
9.2.9. Mylan (Viatris)
9.2.10. Dr. Reddy's Laboratories Ltd.
9.2.11. Qilu Pharma
9.2.12. Taiji Group
9.2.13. Helsinn Group
9.2.14. Heron Therapeutics Inc.
9.2.15. Kyowa Kirin Co., Ltd.
9.2.16. Eisai Co., Ltd.
9.2.17. Aurobindo
9.2.18. F. Hoffmann-La Roche Ltd.
9.2.19. Pharma Ltd.
9.2.20. Other Prominent Players
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.