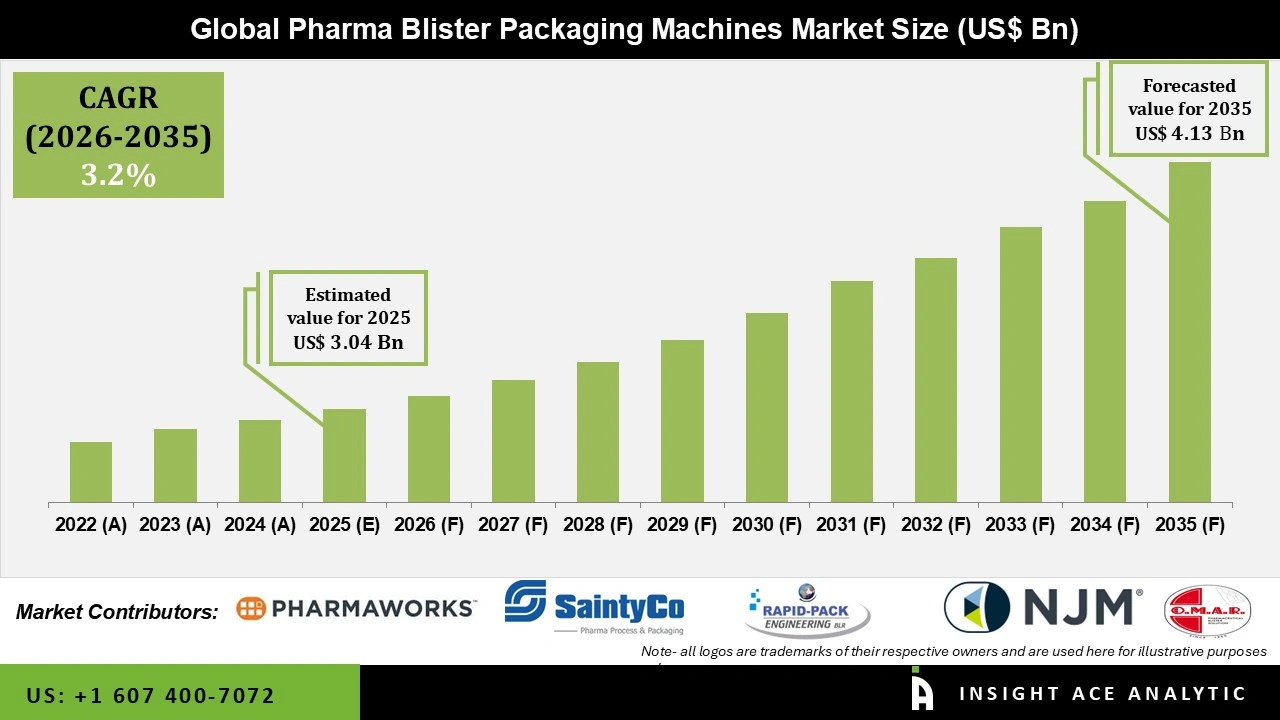
Global Pharma Blister Packaging Machines Market Size is valued at USD 3.04 Bn in 2025 and is predicted to reach USD 4.13 Bn by the year 2035 at a 3.2% CAGR during the forecast period for 2026 to 2035.
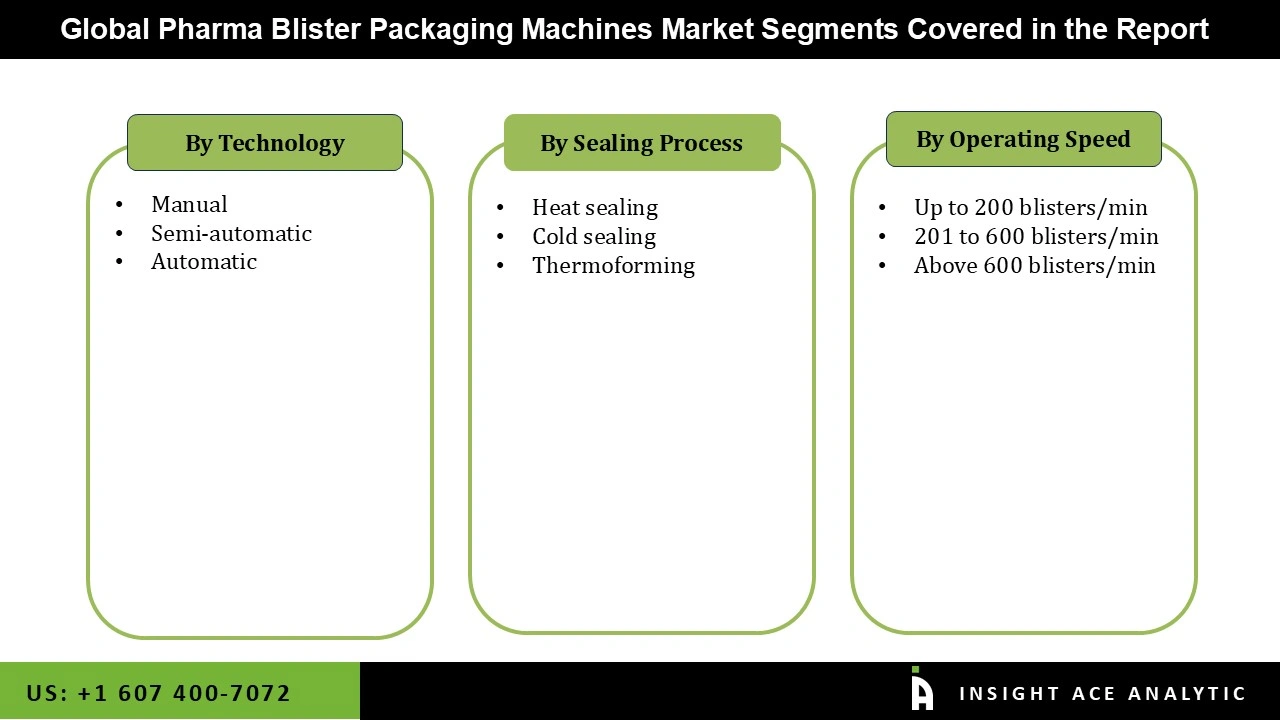
Pharma Blister Packaging Machines Market Size, Share & Trends Analysis Distribution by Sealing Process (Heat Sealing, Cold Sealing, and Thermoforming), Operating Speed (Up to 200 blisters/min, 201 to 600 blisters/min, and Above 600 blisters/min), Technology (Manual, Semi-automatic, and Automatic), and Segment Forecasts, 2026 to 2035.
Pharma blister packaging machines are specialized equipment used in the pharmaceutical industry to create blister packs for tablets, capsules, and other solid medicines. These machines form plastic sheets into individual pockets (blisters), fill them with the product, and seal them with foil or paper backing in a fast, automated process. Blister packs protect drugs from moisture, air, light, and contamination, keeping them stable and effective for a long shelf life. They also provide clear tamper-evidence (easy to see if the pack has been opened) and make it simple for patients to take one dose at a time.
The market for these machines is growing steadily because pharmaceutical companies need safe, reliable, and efficient packaging solutions that meet strict global regulations (GMP standards) and protect product quality. As drug production increases worldwide and patient safety becomes even more important, modern blister machines with higher speed, better accuracy, and features like serialization (anti-counterfeiting) and child-resistant designs are in high demand.
Furthermore, as chronic disease rates rise and the use of oral solid dosage forms increases, pharmaceutical production volumes rise as well, driving up need for automated and high-speed blister packing machines. The rapid growth of generic medication production, especially in developing nations like India and other Asian nations, is hastening adoption even further since producers need dependable and reasonably priced packaging.
In addition, pharmaceutical businesses are being encouraged to transition to sophisticated blister packaging technologies with serialization, inspection, and leak-detection systems due to stringent regulatory requirements pertaining to patient safety, medicine traceability, and tamper-evident packaging. In order to increase production and reduce material waste, manufacturers are implementing robotic feeding systems, integrated visual inspection, and servo-driven machinery. These technical developments are also changing the market. The proliferation of contract manufacturing organizations (CMOs) and the growing need for senior-friendly and child-resistant packaging are also driving the pharma blister packaging machines market expansion. However, the pharma blister packaging machines adoption may be limited, especially among small and mid-sized enterprises, due to the high initial investment cost of automated blister packaging machinery and the requirement for qualified operators.
Driver
Rapid Development in Personalized Medicine
The need for flexible blister packaging machines is increasing due to the advent of personalized medicine, which necessitates small batch outputs and customized packaging. The pharma blister packaging machines market is expected to increase in the near future due to machines made for rapid switchovers and product type adaptation. Customizing therapies for specific patients or small groups with comparable traits is known as personalized medicine. Packaging solutions that can effectively manage smaller, tailored batches are needed for this shift. Additionally, there will be a greater need for blister packing equipment that are adaptable and have fast turnaround times. Precise medication tracking and tracing are essential to personalized therapy. While preserving product integrity, blister packing machines with serialization and track-and-trace features guarantee regulatory compliance. Furthermore, to satisfy the various needs of personalized medication, including labeling, dose-specific packaging, and patient-specific data, blister packaging equipment with automated, high-precision customization will be crucial.
Restrain/Challenge
High Operating Costs and Capital Expenditures
The adoption of pharma blister packaging machines for medicines requires a significant investment because digital printing features, automation, and inspection technologies are required. These extra functionalities might not be economically possible for mid- and small-sized pharmaceutical companies that compete in fiercely competitive and cost-sensitive sectors. Additionally, the rotating maintenance, operator acquisition and training, and adherence to pertinent legal frameworks are additional crucial costs that raise the total cost of ownership. These financial constraints are probably going to hamper the uptake of innovative technologies, particularly among low-volume packagers and in poor nations. Furthermore, the bulk of blister packing equipment is designed for solid oral dosage forms, such as tablets and capsules. However, it may be necessary to package injectable medications and other liquid or powder medications that are sensitive to moisture in vials, ampoules, or sachets, which are more appropriate. These further restrict the spectrum of pharmaceutical goods that can be packaged using blister packaging equipment.
The heat sealing category held the largest share in the Pharma Blister Packaging Machines market in 2025 because it is essential for making sure that oral solid dosage forms are packaged in an airtight, tamper-evident, and contamination-free manner. Aluminum foil or lidding material is frequently bonded with thermoformed plastic cavities using heat sealing technology, forming a safe enclosure that shields tablets and capsules from light, air, and moisture. The need for trustworthy sealing procedures has increased significantly as pharmaceutical businesses place a greater emphasis on patient safety, prolonged shelf life, and drug stability. Furthermore, the growing need for packaging line throughput due to the production of generic and over-the-counter (OTC) medications is pushing manufacturers to incorporate high-precision heat sealing stations into automated blister packaging machines.
In 2025, the 201 to 600 blisters/min category dominated the Pharma Blister Packaging Machines market. Its ideal ratio of throughput, format flexibility, and cost effectiveness accounts for its popularity. Medium- to large pharmaceutical enterprises frequently use machines in this range in order to achieve steady production levels without having to pay the hefty capital expenses associated with ultra-high-speed machinery. These systems frequently facilitate modular upgrades, rapid tool changes, and interaction with inspection systems, allowing producers to react quickly to market needs and the proliferation of SKUs. Additionally, the speed bracket is appropriate for both local and international supply chains since it corresponds with the batch-size variability observed in both the prescription and over-the-counter medicine segments.
The Pharma Blister Packaging Machines market was dominated by North America region in 2025 driven by strict regulations, a firmly established pharmaceutical industry, and widespread use of cutting-edge packaging technologies. Because of the region's emphasis on patient safety, product integrity, and regulatory compliance, blister packaging solutions are widely used.
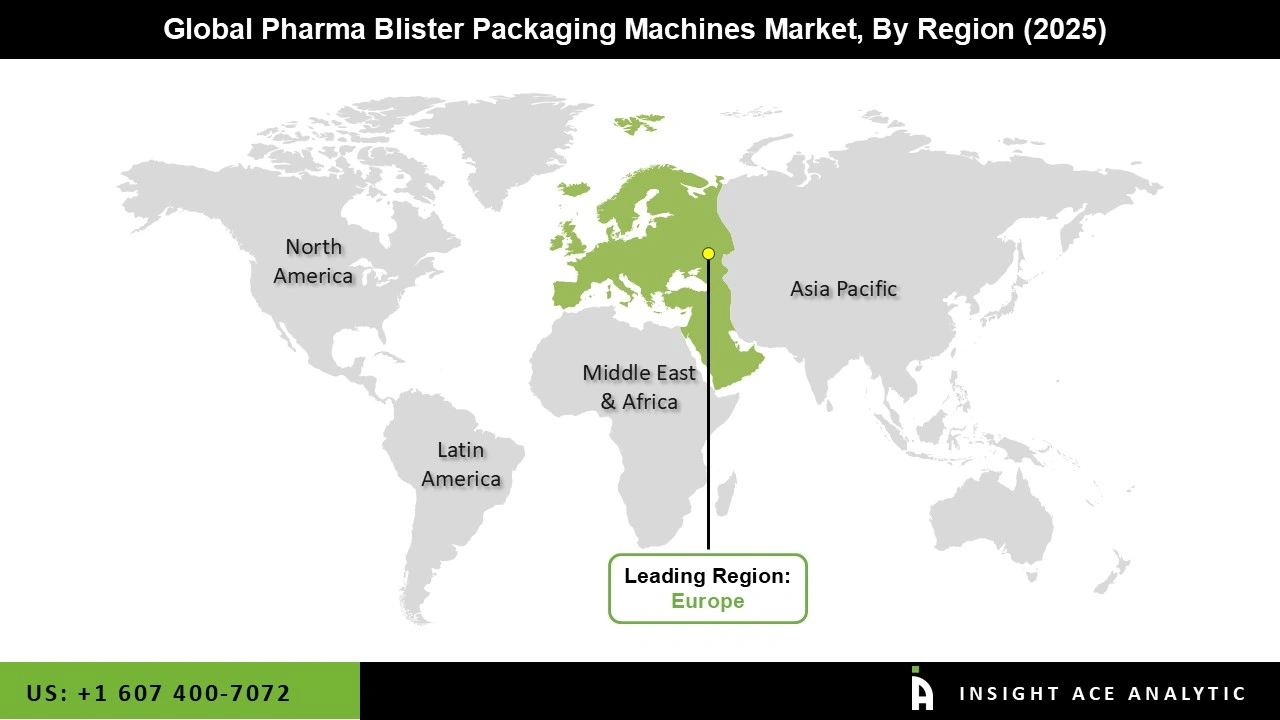
Additionally, the pharmaceutical industry's continued investments and the rising need for creative packaging solutions are likely to support the North American pharma blister packaging machines market's stable growth. Furthermore, the pharma blister packaging machines market is growing because of the presence of major pharmaceutical corporations and contract packaging organizations in North America. The favorable government initiatives and the growing presence of global pharmaceutical companies are expected to promote the market's significant growth in this region.
January 2024: The world's first pharmaceutical-grade blister film containing 30% post-consumer recycled (PCR) content was introduced by TekniPlex Healthcare and Alpek Polyester. This cutting-edge blister film, known as Octal rDPET, helps pharmaceutical businesses comply with new international sustainability standards that aim to lessen the environmental impact of packaging across all sectors.
| Report Attribute | Specifications |
| Market size value in 2025 | USD 3.04 Bn |
| Revenue forecast in 2035 | USD 4.13 Bn |
| Growth Rate CAGR | CAGR of 3.2% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | Sealing Process, Operating Speed, Technology, and By Region |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; Southeast Asia |
| Competitive Landscape | Pharmaworks LLC, Körber AG, Heino Ilsemann GmbH, Mediseal GmbH, O.M.A.R. S.r.l., American Pharma Machinery, Titan Pharmaceutical Machinery, IWKA PacSystems, NJM Packaging, CN International Co. Ltd., SaintyCo., Jornen Machinery Co., Ltd., Nikhil Pharma Packages Company, Romaco Group, Zhejiang Capsul, Aligned Machinery, Hangzhou Shengde Machinery Co., Ltd., LeadTop Pharmaceutical Machinery, Rapid Pack Engineering BLR, Accupack Engineering Pvt. Ltd., and Zhejiang Jiangnan Pharmaceutical Machinery Co., Ltd. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.