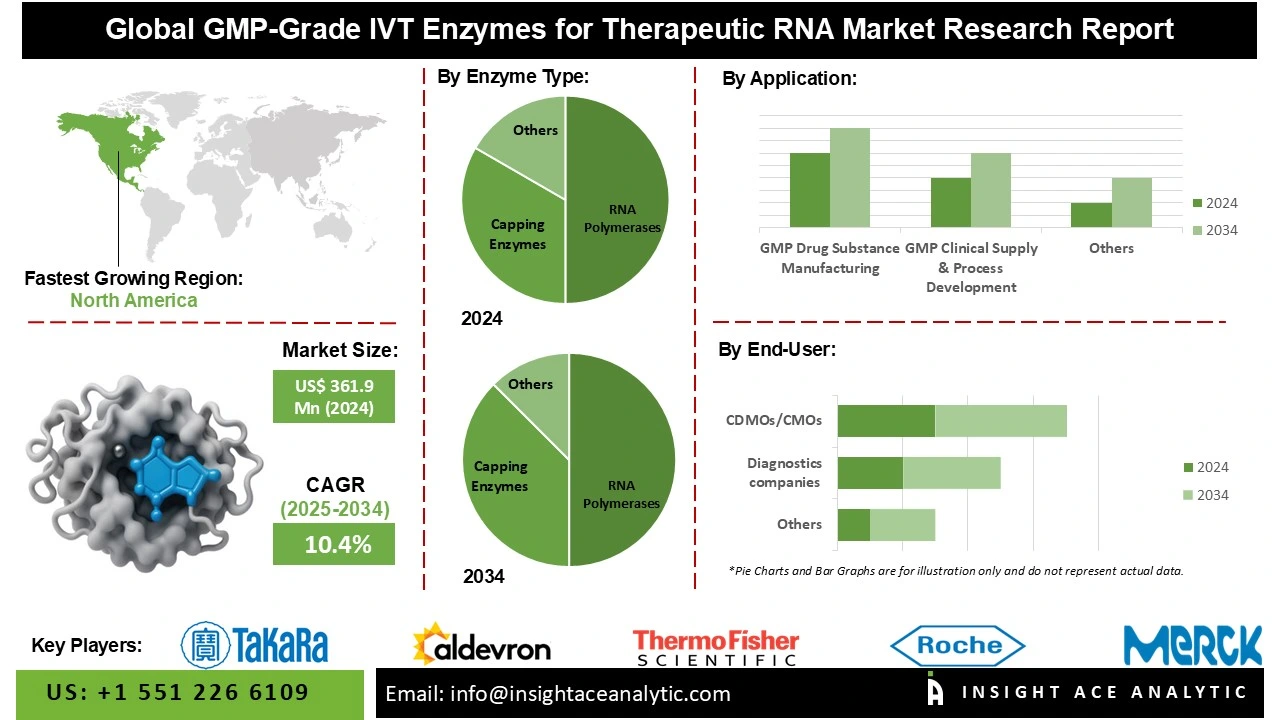
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market Size is valued at USD 361.9 Mn in 2024 and is predicted to reach USD 923.0 Mn by the year 2034 at a 10.4% CAGR during the forecast period for 2025 to 2034.
GMP-Grade IVT Enzymes for Therapeutic RNA Market, Share & Trends Analysis Report, By Enzyme Type (IVT Workflow) (RNA Polymerases, Capping Enzymes, Tailing Enzymes - Poly(A) Polymerase, Template Generation Enzymes, Cleanup / Yield / Protection, Circular RNA Enzymes), By RNA Modality (mRNA, saRNA/replicons, circRNA, gRNAs/crRNAs (CRISPR), Other therapeutic RNAs), By Application / Use Stage (GMP Drug Substance Manufacturing, GMP Clinical Supply & Process Development (Phase I–III), Diagnostics/IVD, Translational Research with GMP continuity), By End User, By Region, and Segment Forecasts, 2025 to 2034
GMP-grade IVT (In Vitro Transcription) enzymes are essential for producing therapeutic RNA including mRNA used in vaccines, Gene therapies, and other RNA-based treatments. Manufactured under strict Good Manufacturing Practice (GMP) standards, these high-purity enzymes ensure consistent, high-quality RNA synthesis suitable for research, preclinical, and clinical applications.
RNA is transcribed from a DNA template containing a T7 promoter, enabling high-yield production commonly used in large-scale manufacturing of mRNA vaccines (such as COVID-19 vaccines), gene-editing tools like CRISPR, and protein replacement therapies. Key GMP-grade IVT enzymes include T7 RNA polymerase, poly(A) polymerase, and capping enzymes such as Vaccinia Capping Enzyme, all of which contribute to generating stable, translation-ready mRNA. Beyond vaccines, these enzymes also support regenerative medicine by enabling the creation of mRNA that drives tissue repair and cellular reprogramming.
Enzymes that enable high-yield, scalable IVT (In Vitro Transcription) processes for producing mRNA at gram-to-kilogram scales are vital to the GMP drug substance manufacturing (commercial) segment. This demand was clearly demonstrated during the COVID-19 pandemic, when billions of vaccine doses required large-scale mRNA synthesis. Among these, GMP-grade T7 RNA polymerase plays a central role engineered for superior activity and stability, it allows manufacturers to consistently meet industrial-scale requirements. Expanded GMP production infrastructure and the growing capabilities of contract manufacturing organizations (CMOs) have further increased the availability of these RNA polymerases, driving the expansion of commercial mRNA production. As the mRNA therapeutics market continues to grow, demand for GMP-grade IVT enzymes rises correspondingly, with RNA polymerases maintaining the largest market share due to their pivotal role in transcription. Continuous advancements in enzyme engineering are now enhancing yield, purity, and cost efficiency, solidifying their importance in large-scale mRNA drug substance manufacturing.
Some of the Major Key Players in the GMP-Grade IVT Enzymes for Therapeutic RNA Market is:
· New England Biolabs (NEB)
· Thermo Fisher Scientific
· Roche CustomBiotech
· Merck KGaA (MilliporeSigma)
· Aldevron (Danaher/Cytiva)
· TriLink BioTechnologies (Maravai)
· Kactus Bio
· Yeasen Biotech
· Takara Bio
· Canvax Biotech
· LGC Biosearch Technologies
· Novoprotein
· Jena Bioscience
· Baseclick GmbH
· Tinzyme
· Promega Corporation
· Kaneka Eurogentec
· BOC Sciences
· Creative Biogene
· HONGENE
The GMP-grade IVT enzymes for the therapeutic RNA market are segmented into enzyme type (IVT workflow), RNA modality, application/use stage, and end user. Based on the enzyme type, the market is segmented into RNA polymerases, capping enzymes, tailing enzymes - poly(A) polymerase, template generation enzymes, cleanup/yield/protection, and circular RNA enzymes. Based on the RNA modality, the market is divided into mRNA, saRNA/replicons, circRNA, gRNAs/crRNAs (CRISPR), and other therapeutic RNAs. Based on the application/use stage, the market is divided into GMP drug substance manufacturing, GMP clinical supply & process development (phase I–III), diagnostics/IVD, and translational research with GMP continuity. Based on the end-user, the market is divided into biopharma/biotech sponsors (in-house manufacturing), CDMOs/CMOs, diagnostics companies, and academic/government translational centers.
mRNA is being widely explored for applications such as protein replacement therapies and cancer immunotherapies, including personalized neoantigen vaccines. To ensure safety, purity, and consistency, all mRNA Therapeutics intended for human use must be produced under rigorous Good Manufacturing Practice (GMP) standards, following guidelines like ICH Q7 and ISO 13485. GMP-grade IVT enzymes such as T7 RNA polymerase, RNase inhibitors, and capping enzymes serve as critical raw materials that meet these regulatory requirements. As more mRNA candidates advance into clinical development, the demand for GMP-grade enzymes has surged; however, limited availability of high-quality raw materials and the high cost of key enzymes like T7 RNA polymerase and DNase I have created supply challenges. The inherent flexibility of the mRNA platform allowing rapid sequence modifications without altering the core production process makes it ideal for fast-response vaccines and personalized therapies, further amplifying the demand for GMP-grade IVT enzymes to ensure reliable, high-quality mRNA synthesis across diverse therapeutic areas.
RNA polymerases are key enzymes in the synthesis of mRNA used for a range of applications, including CRISPR/Cas9 systems that encode Cas proteins and protein replacement therapies targeting conditions such as hemophilia and cystic fibrosis. The expanding pipeline of RNA-based therapeutics is driving strong demand for high-quality, GMP-grade RNA polymerases to ensure a consistent supply for both clinical and commercial-scale production. Within the therapeutic RNA ecosystem, the RNA polymerase segment plays a crucial role in overcoming supply chain bottlenecks by providing reliable, high-purity enzymes. Ongoing advances in RNA polymerase engineering aimed at improving transcription efficiency and minimizing unwanted double-stranded RNA byproducts are further strengthening supplier competitiveness. Meanwhile, emerging technologies such as microfluidics and continuous IVT systems are enhancing the scalability and productivity of RNA synthesis, fueling the growing demand for GMP-grade RNA polymerases to support next-generation RNA therapies.
North America dominates the market for GMP-grade IVT enzymes for therapeutic RNA due to significant investments from private investors and pharmaceutical companies that are speeding up the development of mRNA technology, as well as significant financing from government agencies like the NIH and BARDA. This financial assistance directly supports the need for high-quality GMP-grade enzymes essential for both clinical and commercial-scale RNA synthesis. In May 2024, U.S.-based Aldevron joined forces with Acuitas Therapeutics to enhance mRNA services through lipid nanoparticle (LNP) encapsulation, further solidifying this leadership. The partnership combines state-of-the-art LNP formulation with GMP-grade RNA polymerases, such as Aldevron's Codex® HiCap T7 RNA Polymerase, to create an integrated solution that expedites the manufacturing of mRNA therapeutics and further solidifies North America's leadership in providing end-to-end mRNA platforms.
· In April 2024, TriLink BioTechnologies (TriLink), a Maravai LifeSciences company declared its new cGMP mRNA production facility's grand opening. Using TriLink's strong mRNA production capabilities, the 32,000-square-foot facility was built especially for mRNA manufacture to assist late-phase drug researchers from Phase 2 to commercialization. As developers swarm to capitalize on the promising technique for an expanding range of applications, the milestone opening is anticipated to contribute to the advancement of mRNA-based therapy. The facility, which is situated in San Diego's Sorrento Valley, has separate Grade C cleanroom suites for the production of mRNA, a capacity increase from 1g to >100g per batch, extensive in-house analytical capabilities, and laboratory space for on-site quality control testing.
· In Mar 2023, Creative Biogene was committed to improving the newest medical technologies, such as vaccines, gene editing, cell treatments, and immunotechnology. In order to support research in the areas of preclinical drug discovery, industrial synthetic application, biomedical development, and fundamental life sciences research, Creative Biogene delivers knowledge to deliver products of the highest quality consistently on time. To further research and project development across multiple domains, Creative Biogene has announced the launch of its GMP-grade mRNA synthesis services.
| Report Attribute | Specifications |
| Market Size Value In 2024 | USD 361.9 Mn |
| Revenue Forecast In 2034 | USD 923.0 Mn |
| Growth Rate CAGR | CAGR of 10.4% from 2025 to 2034 |
| Quantitative Units | Representation of revenue in US$ Mn and CAGR from 2025 to 2034 |
| Historic Year | 2021 to 2024 |
| Forecast Year | 2025-2034 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Enzyme Type (IVT Workflow), RNA Modality, Application / Use Stage, End User |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; South East Asia |
| Competitive Landscape | New England Biolabs (NEB), Thermo Fisher Scientific, Roche CustomBiotech, Merck KGaA (MilliporeSigma), Aldevron (Danaher/Cytiva), TriLink BioTechnologies (Maravai), Kactus Bio, Yeasen Biotech, Takara Bio, Canvax Biotech, LGC Biosearch Technologies, Novoprotein, Jena Bioscience, Baseclick GmbH, Tinzyme, Promega Corporation, Kaneka Eurogentec, BOC Sciences, Creative Biogene, HONGENE |
| Customization Scope | Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market- By Enzyme Type (IVT Workflow)
· RNA Polymerases
o T7 RNA Polymerase (dominant)
o SP6 RNA Polymerase
o T3 RNA Polymerase
· Capping Enzymes
o Vaccinia Capping Enzyme (Cap-0)
o mRNA Cap 2′-O-Methyltransferase (Cap-1)
o Alternative Viral Capping Enzymes (Faustovirus,etc)
· Tailing Enzymes - Poly(A) Polymerase
· Template Generation Enzymes
o Restriction Endonucleases
o High-fidelity DNA Polymerases
· Cleanup / Yield / Protection
o DNase I / dsDNase (RNase-free)
o Inorganic pyrophosphatase
o RNase inhibitor
· Circular RNA Enzymes
o T4 RNA Ligase I/II
o RNase R
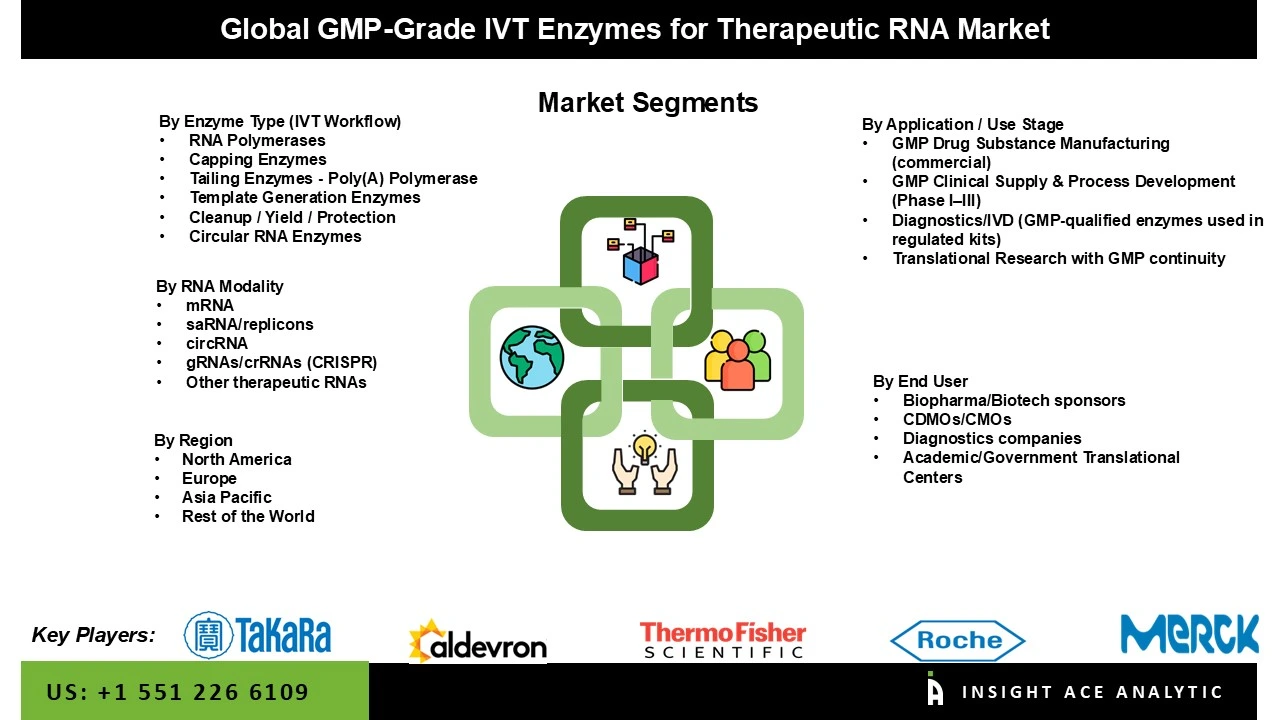
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market – By RNA Modality
· mRNA
· saRNA/replicons
· circRNA
· gRNAs/crRNAs (CRISPR)
· Other therapeutic RNAs (lncRNA/antisense where IVT is used)
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market – By Application / Use Stage
· GMP Drug Substance Manufacturing (Commercial)
· GMP Clinical Supply & Process Development (Phase I–III)
· Diagnostics/IVD (GMP-Qualified Enzymes Used in Regulated Kits)
· Translational Research with GMP continuity (Pilot/Tech-transfer Lots)
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market- By End User
· Biopharma/Biotech sponsors (In-house Manufacturing)
· CDMOs/CMOs
· Diagnostics companies
· Academic/Government Translational Centers
Global GMP-Grade IVT Enzymes for Therapeutic RNA Market – By Region
North America-
· The US
· Canada
Europe-
· Germany
· The UK
· France
· Italy
· Spain
· Rest of Europe
Asia-Pacific-
· China
· Japan
· India
· South Korea
· Southeast Asia
· Rest of Asia Pacific
Latin America-
· Brazil
· Mexico
· Rest of Latin America
Middle East & Africa-
· GCC Countries
· South Africa
· Rest of the Middle East and Africa
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.