Global Intra-abdominal Pressure Measurement Device Market Size is valued at USD 91.86 Million in 2025 and is predicted to reach USD 419.59 Million by the year 2035 at a 17.2% CAGR during the forecast period for 2026 to 2035.
Intra-abdominal Pressure Measurement Device Market Size, Share & Trends Analysis Report By Procedure (Muscle, Abdomen), By Product Scope (Disposables, Equipment), By Application Scope, By End-User, By Region and Segment Forecasts, 2026 to 2035
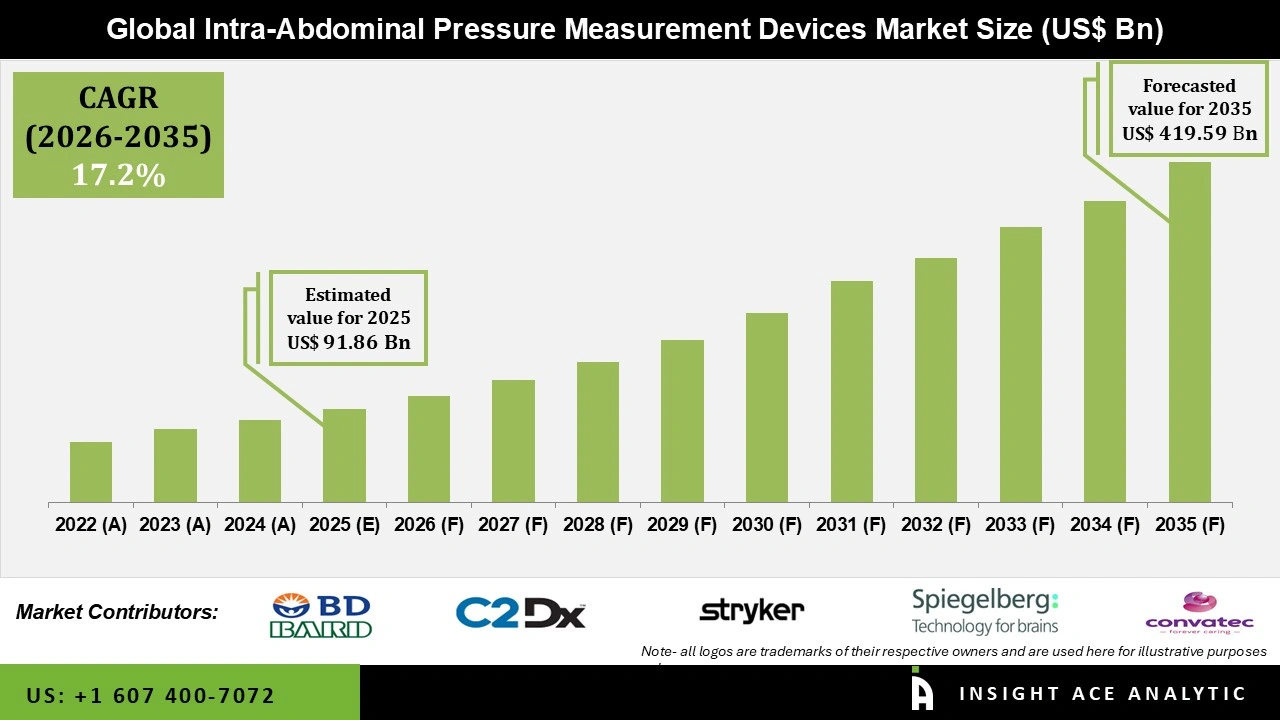
Key Industry Insights & Findings from the Report:
Intra-abdominal hypertension is defined as a blood pressure of more than 12 millimetres of mercury, which is the threshold at which organ dysfunction develops. The market is growing because of the rising incidence rate of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS). The increasing number of patients in critical care units has a positive influence on the market. Additionally, rising R&D efforts, government initiatives to utilize sustainable components in manufacturing, and investments by major companies are likely to generate attractive revenue possibilities for participants in the worldwide intra-abdominal pressure measurement device market throughout the forecast period.
In the case of health instruments and devices, rising research, and development expenditures, particularly in developed and emerging countries, will create even more profitable market growth potential. Research and development capabilities for the creation of pharmaceuticals and other treatment options also contribute to the market's growth pace. On the other hand, market growth is expected to be hampered by high costs associated with research and development capabilities, inadequate infrastructural facilities, and increased complications and concerns around assisted reproductive technology (ART). In addition, in the forecast period, the market is expected to be challenged by a lack of favourable reimbursement scenarios and technology penetration in developing economies, high costs associated with assisted reproductive technology (ART) procedures, and a lack of suitable infrastructure in low- and middle-income countries.
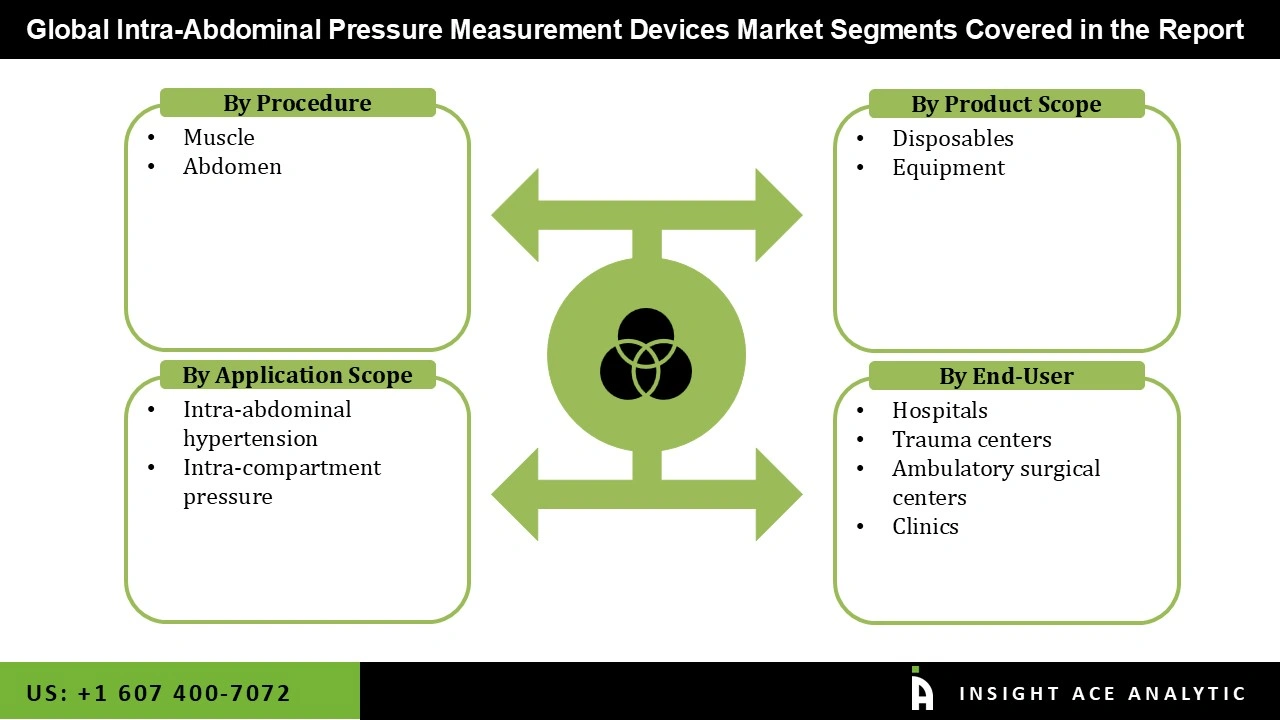
The intra-abdominal pressure measurement device market is categorized based on product, procedure, end-users and application. Based on Product, the market is bifurcated into disposables and equipment, based on procedure the market is segmented into muscle and abdomen), based on application, the market is segmented into intra-abdominal hypertension and intra-compartment pressure and Based on end user, the market is segregated into hospitals, trauma centers, ambulatory surgical centers [ASCs], and clinics.
The abdomen category is expected to hold a significant share in the global intra-abdominal pressure measurement device market in 2024. The segment growth is due to an increase in the number of critically ill individuals who have intra-abdominal hypertension. In such patients, increased intra-abdominal pressure impairs organ function, potentially resulting in abdominal compartment syndrome. As increased intra-abdominal pressure impairs organ function in these patients, abdominal compartment syndrome can develop. Direct needle penetration in the abdominal cavity is used to obtain intra-abdominal hypertension measurements.
The hospitals segment is expected to expand at a rapid rate in the global intra-abdominal pressure measurement device market. This is due to several advancements in developing a simple and practical way of monitoring intra-abdominal pressure that can be set up quickly even at a remote hospital and has been validated in both experimental and clinical research, especially in countries such as the US, Germany, UK, China, and India.
The North America intra-abdominal pressure measurement device market is expected to register the highest market share in terms of revenue soon due to a strong base of healthcare facilities, increasing investment from major players in the growth of advanced devices, an increase in blunt abdominal trauma cases due to motor vehicle accidents, an increase in the number of drug development processes, and an increase in the number of research activities in this region.
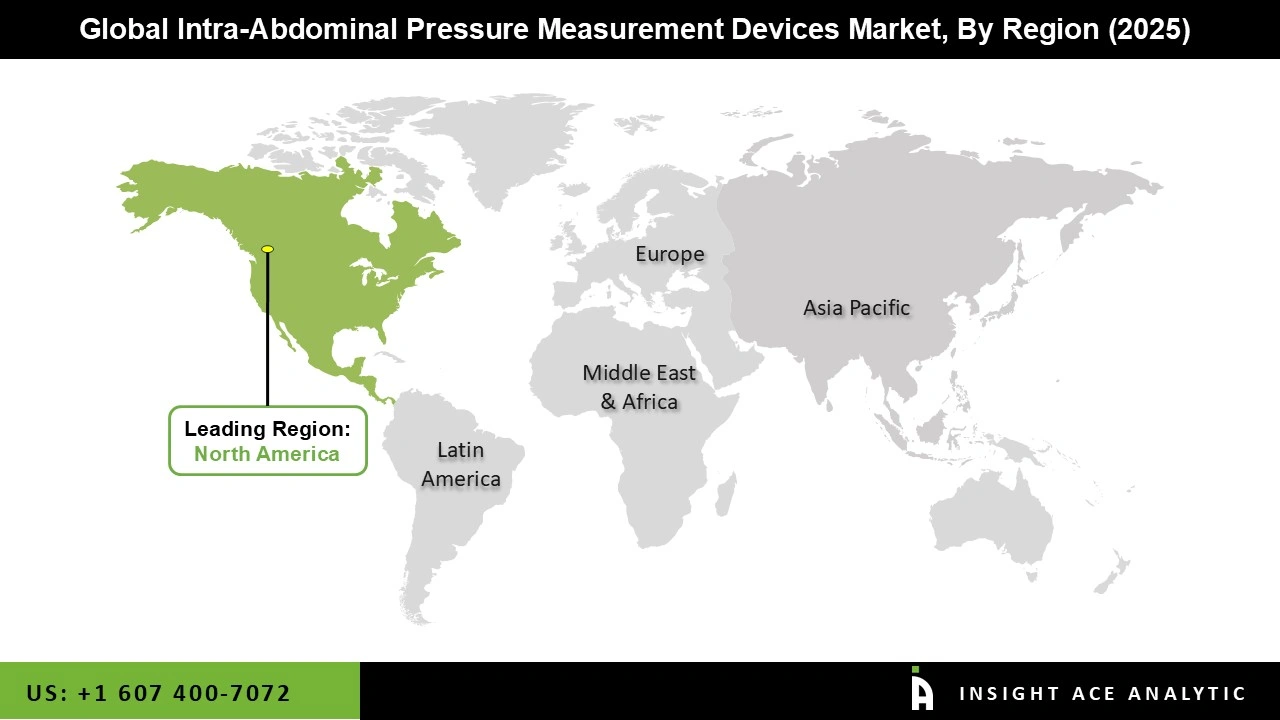
The market in North America is projected to grow because of supportive health care spending, government efforts, and the desire for better treatment. In addition, Asia Pacific is projected to proliferate in the global intra-abdominal pressure measurement devices market due to increased government attempts to raise awareness, an increase in medical tourism, and an increase in research activity in the region.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 91.86 Million |
| Revenue Forecast In 2035 | USD 419.59 Million |
| Growth Rate CAGR | CAGR of 17.2% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Million and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026 to 2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Procedure, By Product Scope, By Application Scope, By End-User |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
| Competitive Landscape | C. R. Bard, Inc, C2Dx Inc, Stryker, Spiegel Berg GmbH & Co. KG, Biomatrix Ltd, Centurion Medical Products (Medline), ConvaTec Group PLC, Gaeltacht Devices Ltd., Potrero Medical Inc, and other prominent player. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.