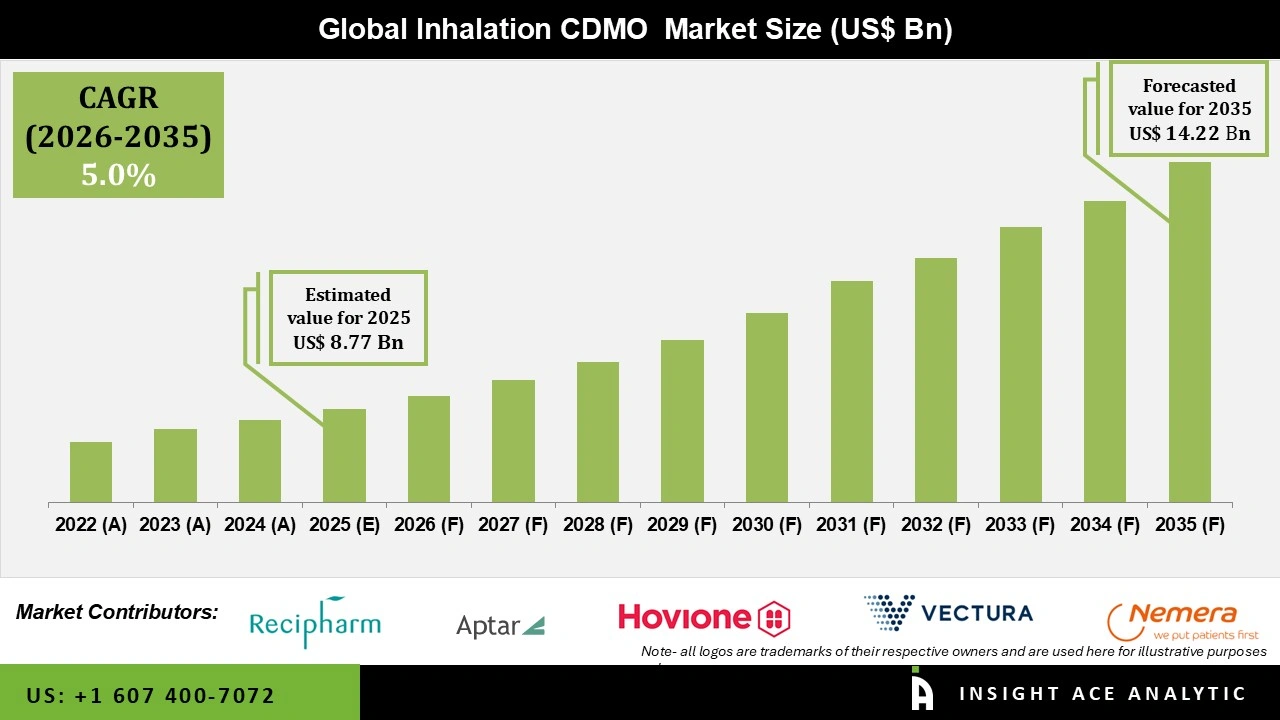
Global Inhalation CDMO Market Size was valued at USD 8.77 Bn in 2025 and is predicted to reach USD 14.22 Bn by 2035 at a 5.0% CAGR during the forecast period for 2026 to 2035.
Inhalation CDMO Market Size, Share & Trends Analysis Report, By Product (API, Inhalation Platform), By Services (Formulation Development, Device Development and Manufacturing, Clinical Manufacturing, Scale-up and Tech Transfer, Quality Control and Quality Assurance, Technology and Innovation, Regulatory Assistance, Analytical Services), By Company Size, By Scale of Operation, By Region and Segments Forecasts, 2026 to 2035.
Inhalation CDMO refers to the organization that is responsible for the development and manufacturing of inhalation contracts. For the research and production of inhalation medicinal products, these specialized service providers offer solutions that cover the entire continuum. One of the main factors fueling the growth of the inhalation CDMO market is the increasing number of respiratory disorders globally. Air pollution, an aging population, and increased urbanization are contributing to the alarming increase in chronic obstructive pulmonary disease, cystic fibrosis, and asthma cases.
The inhalation CDMO industry is leading the way in technological innovation, improving inhalation medicines' safety, effectiveness, and patient experience by utilizing new ideas in aerosol science, device design, and manufacturing processes. Furthermore, the inhalation CDMO market has a lot of potential due to factors such the growing need for novel inhalation medicines, improvements in drug delivery technologies, and the trend of pharmaceutical companies outsourcing more and more to simplify production and development.
However, the market growth is hampered due to complex formulation procedures, high development and manufacturing expenses, and strict regulatory restrictions. Smaller businesses find it challenging to break into a market due to the high operational costs caused by the requirement for specialized equipment and experience. The COVID-19 pandemic has sped up the inhalation CDMO market because of higher demand for respiratory treatments, shorter lead times for drug development, and a greater focus on advanced drug delivery methods. This has led to more investment and new ideas in the inhalation CDMO market. Furthermore, rising collaborations between pharmaceutical firms and CDMO to address critical healthcare demands, faster drug approval processes, and heightened attention to respiratory health all contributed to the market's meteoric rise.
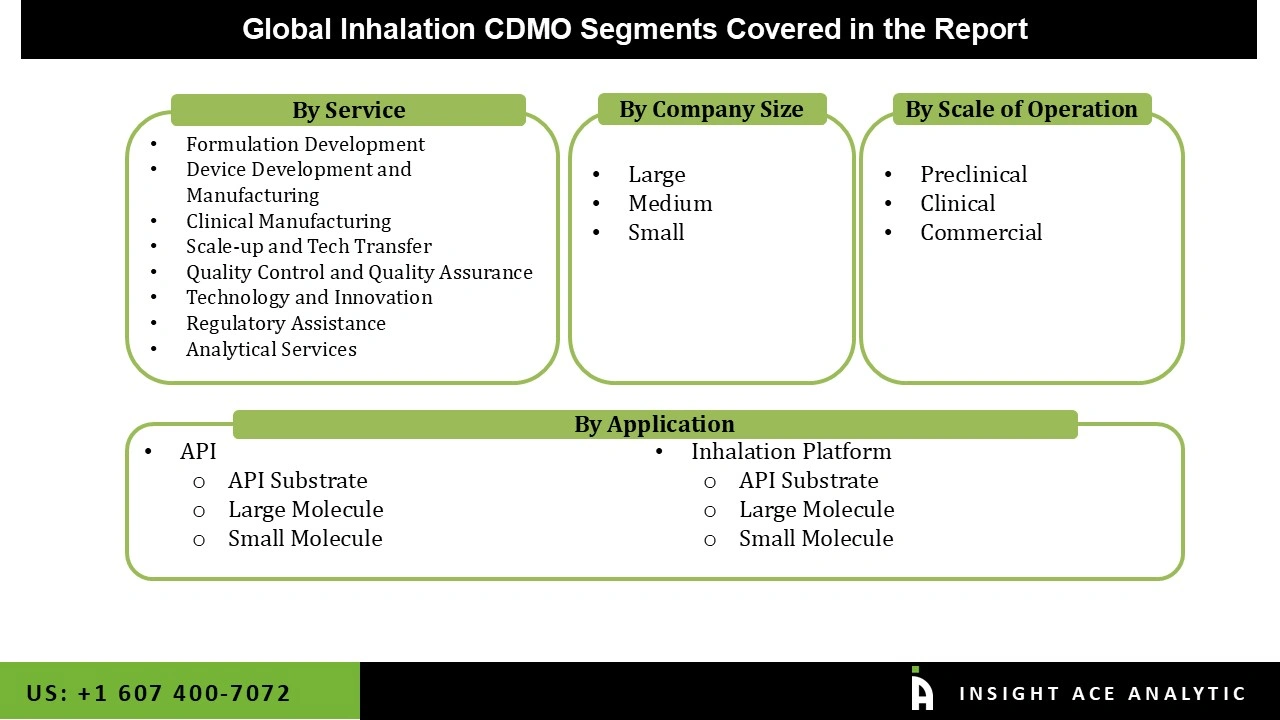
The global inhalation CDMO market is segmented based on service, product, company size and scale of operation. Based on service, the market is segmented into formulation development, device development and manufacturing, clinical manufacturing, scale-up and tech transfer, quality control and quality assurance, technology and innovation, regulatory assistance, and analytical services. By product, the market is segmented into API and inhalation platforms. The market is segmented by company size into large, medium, and small. By scale of operation, the market is segmented into preclinical, clinical, and commercial.
The formulation development segment is expected to hold a major global market share in 2023 because new and improved inhalation treatments are in high demand. A lot of investment and effort has gone into developing effective drug delivery methods, assuring medication stability, and meeting demanding regulatory standards. To achieve these goals, advanced formulation processes are essential, which in turn drives their widespread use and substantial market share.
The inhalation platform segment in the inhalation CDMO market is growing because there is a growing need for focused and effective drug delivery systems for respiratory disorders. This market is experiencing rapid innovation due to technological advancements like dry powder inhalers and metered-dose inhalers. The demand for safe and effective inhalation treatments is another factor driving the expansion of inhalation platforms.
The North American inhalation CDMO market is expected to register the highest market share in revenue in the near future. This is because there is a robust healthcare system, many people suffer from respiratory illnesses, a lot of money spent on research and development, and a powerful pharmaceutical sector.
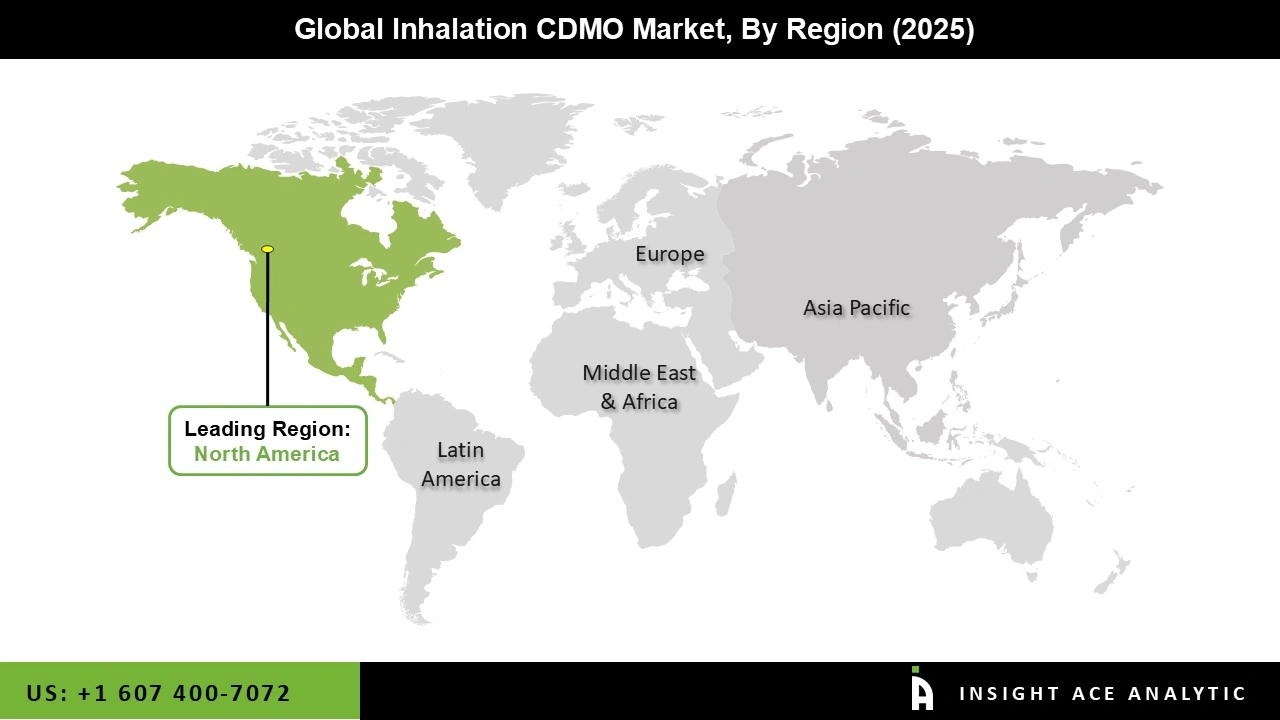
Major inhalation businesses are located in the region, which helps fuel the expansion of the market. In addition, Asia Pacific is projected to grow rapidly in the global inhalation CDMO market as a result of its burgeoning pharmaceutical industry, skyrocketing healthcare costs, increasing number of cases of respiratory disorders, and skyrocketing spending in R&D. Additionally, market expansion in the region is being propelled by a big patient population and supportive government efforts.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 8.77 Bn |
| Revenue Forecast In 2035 | USD 14.22 Bn |
| Growth Rate CAGR | CAGR of 5.0% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$Bn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Service, By Application, By Company Size, By Scale of Operation |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; France; Italy; Spain; South East Asia; South Korea |
| Competitive Landscape | Recipharm AB, AptarGroup Inc., Hovione, Vectura Group Ltd, Nemera, Kindeva, H&T Presspart, Sanner GmbH, Stevanato Group, Medspray, ICONOVO AB, Lonza, Gerresheimer AG, Catalent, Thermo Fisher Scientific Inc., Lubrizol Life Science, Enteris BioPharma, Cambrex Corporation, INKE, Piramal Pharma Limited, Lupin, and Others. |
| Customization Scope | Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.