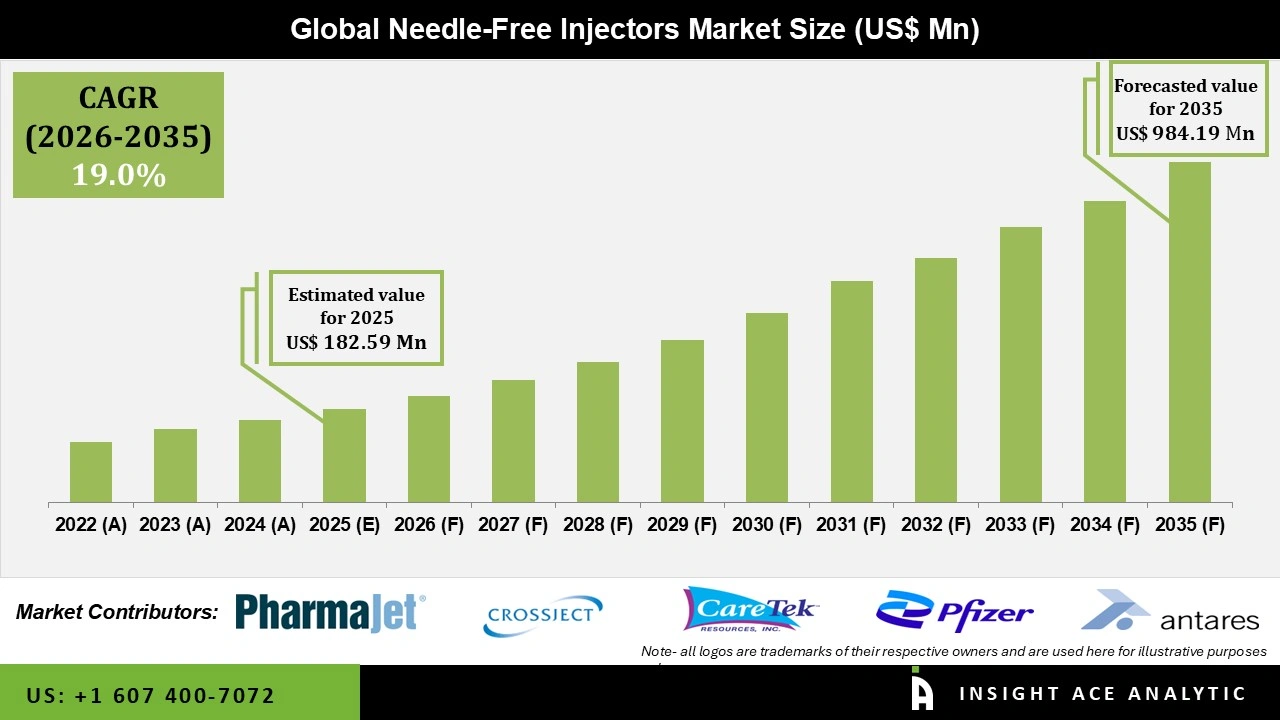
Global Needle-Free Injectors Market Size is valued at USD 182.59 Million in 2025 and is predicted to reach USD 984.19 Million by the year 2035 at a 19.0% CAGR during the forecast period for 2026 to 2035.
Needle-Free Injectors Market Size, Share & Trends Analysis Report By Applications (Drug Delivery, Oncology, Pain Management, Vaccine delivery, Cosmetic, Other Applications) By Type, By Technology, By Product, By End-Users, By Delivery Site, By Usage, Region and Segment Forecasts, 2026 to 2035.
Needle-free injectors are an innovative way to introduce various medicines into patients without piercing the skin with a traditional needle. Needle-free injection systems are safer, effective, and more suitable as compared to conventional needles. These systems are available in different forms, such as power sprays, edible products, inhalers, and skin patches. Usage of these injectors can reduce the number of needle stick accidents and associated problems.
The growing demand for self-injection devices, advanced medical infrastructure, growing geriatric population, rising prevalence of chronic and infectious diseases, lack of reusability of traditional needles, increasing healthcare expenditures, and the rising need for vaccination are the major driving factors of the needle-free injectors market. Pharmaceutical companies focus on developing advanced, safe, and pain-free injectors to fulfill the growing demand for self-injection devices. Therefore, launching new quality products in the market is expected to fuel the growth of the needle-free injectors market during the forecast period. However, the high manufacturing cost of needle-free injections and the lack of trained personnel are likely to limit the market growth during the forecast period.
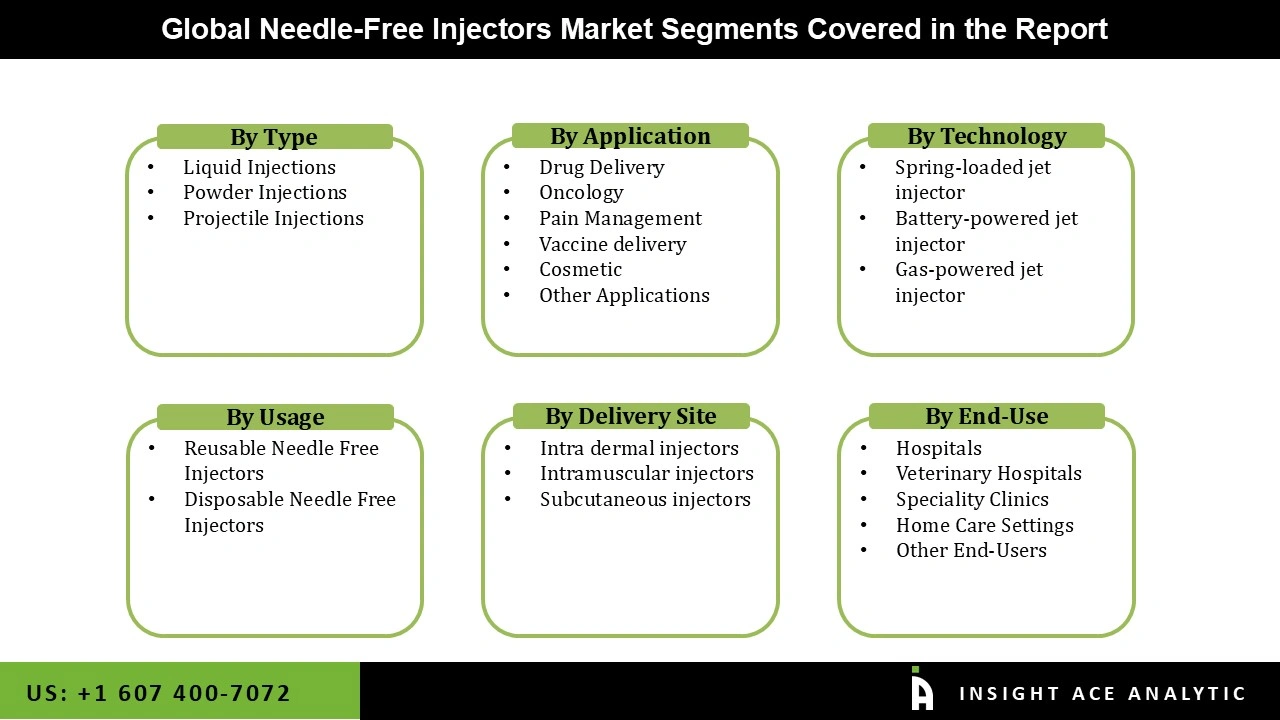
The needle-free injectors market is classified based on applications, load, technology, end-users, delivery site, usage, and region. The applications segment comprises drug delivery, vaccines, cosmetics, and other applications. The drug delivery segment is expected to dominate this market in the near future due to the rising chronic diseases and geriatric population. By load, the market is segmented into liquid injections, powder injections, projectile injections. The liquid segment is anticipated to hold a significant market share in the coming days due to its rapid administration and flexible nature. By technology, the market is classified into spring-loaded jet injectors, battery-powered jet injectors, gas-powered jet injectors. The market comprises hospitals, veterinary hospitals, specialty clinics, home care settings, and other end-users based on end-users.
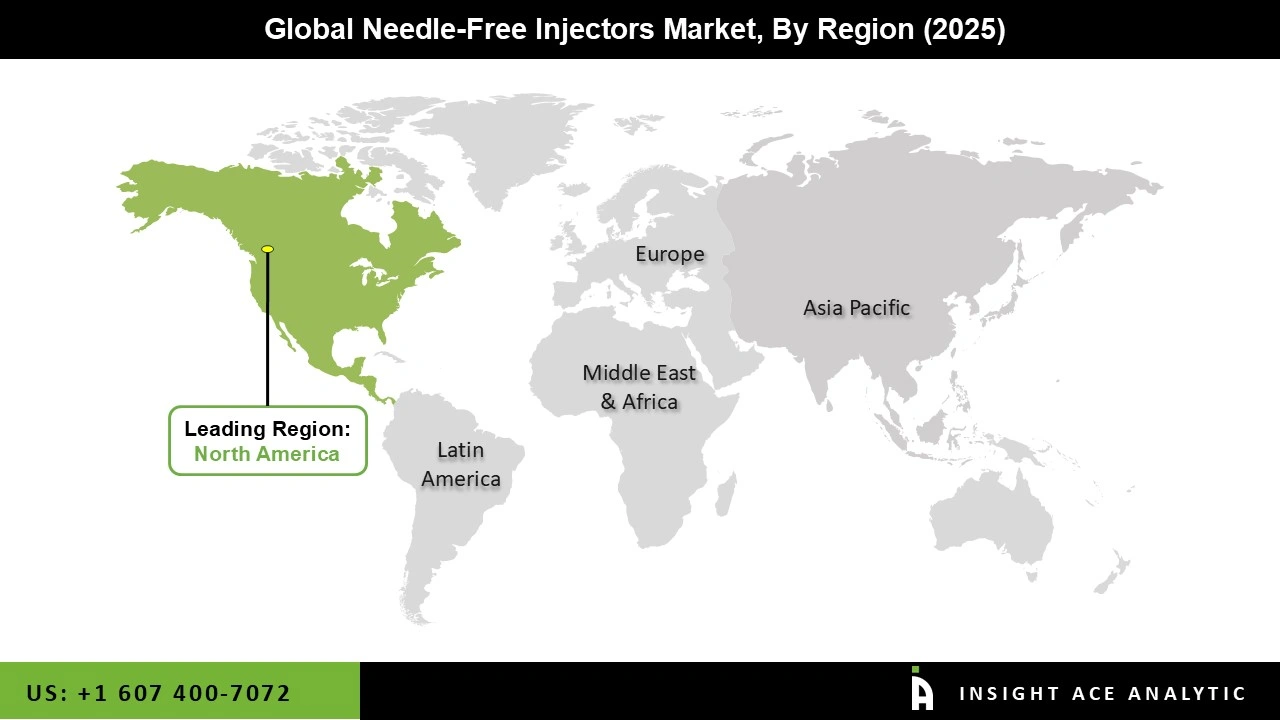
The market is grouped into intradermal injectors, intramuscular injectors, and subcutaneous injectors in terms of delivery site. The subcutaneous injectors segment is expected to grow rapidly over the forecast years due to its speed and low cost. Based on the usage, the market is segmented into reusable needle-free injectors and disposable needle-free injectors. Geographically, the market is studied across North America, Europe, Asia-Pacific, Latin America, and the Middle East, and Africa. North America is expected to hold the highest share of this market during the forecast period, followed by Europe due to the high prevalence of chronic diseases and the advancement of medical technologies.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 182.59 Million |
| Revenue Forecast In 2035 | USD 984.19 Million |
| Growth Rate CAGR | CAGR of 19.0% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Million, Volume (Unit), and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Applications, By Type, By Product, By Technology, By End-Users, By Delivery Site, By Usage |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
| Competitive Landscape | Pfizer (US), Antares Pharma, Inc. (US), Injex UK (UK), Gaungzhou Medsinglong Medical Equipment Company Ltd. (China), Zogenix (US), D'Antonio Consultants International, Inc. (US), AcuShot Needle Free (Canada), InsuJet (The Netherlands), QS Medical Technology Co., Ltd (China), MIKA MEDICAL CO. (South Korea), and Others |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.