Global Advanced Therapy Medicinal Products CDMO Market Size is valued at USD 7.56 Billion in 2025 and is predicted to reach USD 30.25 Billion by the year 2035 at a 15.0% CAGR during the forecast period for 206 to 2035.
Advanced Therapy Medicinal Products CDMO Market Size, Share & Trends Analysis Report By Product (Gene Therapy, Cell Therapy, Tissue Engineered, Others), By Phase, Indication, By Region and By Segment Forecasts, 2026 to 2035
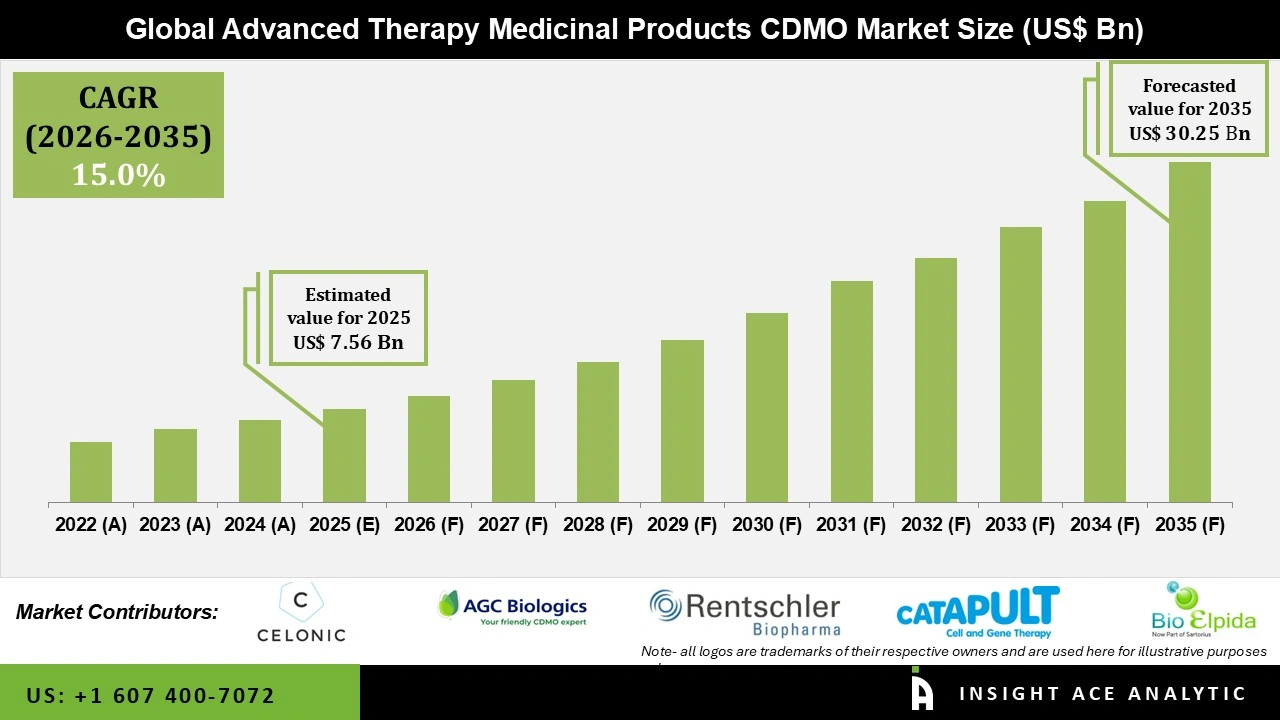
Key Industry Insights & Findings from the Report:
Advanced therapy medicinal products are a class of human-use biological products that include gene therapy, cell therapy, and tissue-engineered products. The expanding clinical studies of ATMP and rising awareness and belief among researchers about the benefits of advanced treatment are associated with the market's expansion. The rising demand for sophisticated therapies is driving market expansion.
The growth is attributable to the increasing occurrence of rare and life-threatening disorders, such as metabolic and ocular diseases, and increased investment in R&D of advanced therapeutic medical goods. Furthermore, ATMPs such as mesenchymal stem cells (MSCs) represent a novel treatment for the COVID-19 virus. Due to the complexity of the manufacturing process, the COVID-19 pandemic has dramatically affected the cell and gene therapy sector. Tissue engineering has significantly benefited from technological advances in recent years. This approach aids in the replacement or restoration of injured tissues and organ function.
Similarly, gene and cell therapy are attracting many patients to treat rare disorders, the prevalence of which is increasing globally. Numerous prominent players use market strategies such as technical alliances and collaborations, mergers and acquisitions, unique product debuts with approvals, R & D activities, strategic initiatives, training services, and regional expansion to build a stronghold in the market growth.
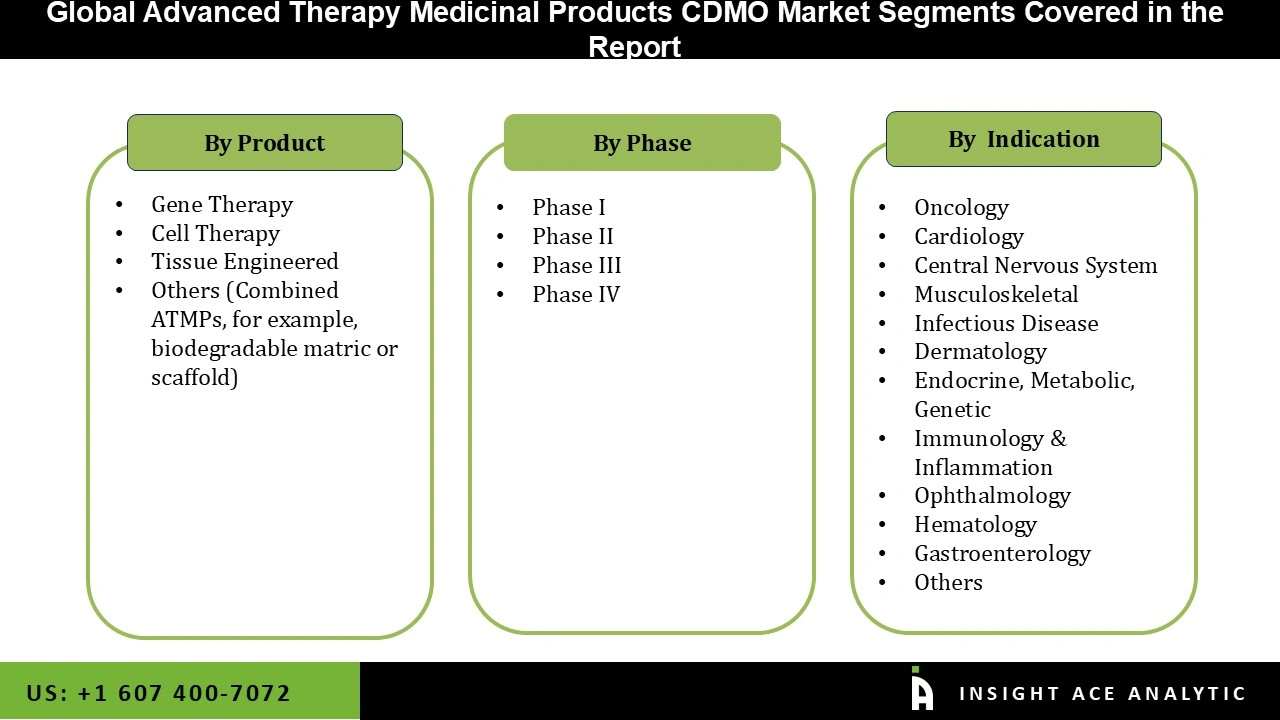
The Advanced Therapy Medicinal Products CDMO market is segmented on the product, phase, and indication. Based on product, the market is segmented into gene therapy, cell therapy, tissue-engineered and others (combined ATMPs, for example, biodegradable matric or scaffold). Based on phase, the Advanced Therapy Medicinal Products CDMO are segmented into phases I, II, III, and IV. Based on the indication, the Advanced Therapy Medicinal Products CDMO are segmented into oncology, cardiology, central nervous system, musculoskeletal, infectious disease, dermatology, endocrine, metabolic, genetic, immunology & inflammation, ophthalmology, hematology, gastroenterology and others.
Based on product, the market is segmented into gene therapy, cell therapy, tissue-engineered and others (combined ATMPs, for example, biodegradable matric or scaffold). The gene therapy segment is expected to account for the highest share of the market. The rapid expansion of the segment can be ascribed to therapeutic breakthroughs since the treatment can alter and improve genetics or modify the targeted treatment. Another aspect driving expansion is increased awareness, which leads to patients wanting this therapy even during the clinical stages. Gene therapy has seen rapid expansion in recent years because of its effectiveness in penetrating cells and beginning genetic materials.
Based on the indication, the Advanced Therapy Medicinal Products CDMO are segmented into oncology, cardiology, central nervous system, musculoskeletal, infectious disease, dermatology, endocrine, metabolic, genetic, immunology & inflammation, ophthalmology, hematology, gastroenterology and others. The oncology segment dominated the market. The increasing incidence of cancer and chronic diseases due to the growing elderly population is contributed to the segment's growth. Oncology is a medical speciality that diagnoses and treats cancer. ATMPs were first used to find a breakthrough in cancer treatment, and as a result, this segment has seen the most expertise and effort over the years.
The North American Advanced Therapy Medicinal Products CDMO market is expected to register the highest market share in revenue shortly. The region is expected to account for the significant share of market revenue. North America's market relevance is achievable due to rising awareness of advanced therapy and expanding outsourcing operations. In addition, the United States has been a pioneer in R&D operations to provide new treatments to the healthcare business. As a result of these factors, the market is expected to grow at a rapid pace in the approaching years.
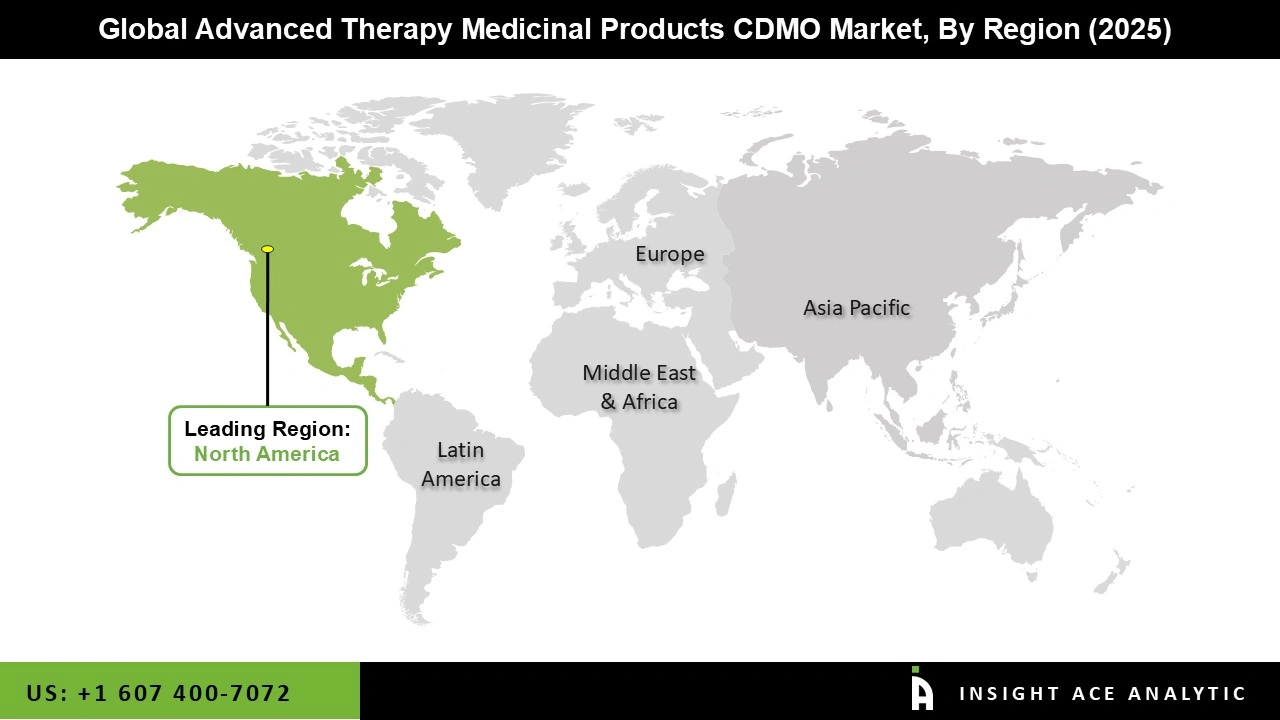
In addition, Asia Pacific is projected to expand rapidly in the global Advanced Therapy Medicinal Products CDMO market because of developments in treatment technology and increased outsourcing activity in the region. The leading competitors in the advanced therapy medicinal products CDMO market are working hard to meet the region's need for advanced therapy therapeutic goods.
| Report Attribute | Specifications |
| Market size value in 2025 | USD 7.56 Billion |
| Revenue forecast in 2035 | USD 30.25 Billion |
| Growth rate CAGR | CAGR of 15.0% from 2026 to 2035 |
| Quantitative units | Representation of revenue in US$ Million and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments covered | Product, Phase, Indication |
| Regional scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country scope | U.S.; Canada; U.K.; Germany; China; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; Japan; India; South Korea; South East Asia |
| Competitive Landscape | Celonic; Bio Elpida; CGT Catapult; Rentschler Biopharma SE; AGC Biologics; Catalent; Lonza; WuXi Advanced Therapies; BlueReg; Minaris Regenerative Medicine; Patheon. |
| Customization scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and available payment methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.