The CAR-T Cell Therapy for Multiple Myeloma Market is expected to grow at a 27.0 % CAGR during the forecast period for 2023-2031.
Chimeric Antigen Receptor (CAR) T cell therapy is an innovative treatment that encompasses the reengineering of white blood cells (WBC) of a cancer patient’s to fight the malignant cells by identifying them. CAR-T cell therapy is approved for certain types of blood cancer. The blood cancer type, multiple myeloma affects plasma cells. In multiple myeloma, the malignant cells accrue in the bone marrow (the spongy, soft tissue at the middle of bones), gathering out the normal plasma cells that help fight contamination. These malicious plasma cells then produce M protein, an atypical antibody that offers no advantage to the body and might cause kidney damage, tumors, bone obliteration, and reduced immune function. The high level of M protein in blood denotes the characteristics of multiple myeloma.
The Multiple Myeloma is infrequently curable, but it is an extremely manageable disease that has seen speedy medical development over the past few years. The development of the Multiple Myeloma Research Foundation (MMRF) has improved the research activities to develop the most promising treatments for multiple myeloma. CAR T-cell therapy for multiple myeloma is one advanced immunotherapy to program the immune system to attack cancer. The CAR-T Cell therapy for multiple myeloma market size is expected to reach US$ xx million by 2030, growing at a CAGR of xx% over the forecast period. The rising prevalence of multiple myeloma is anticipated to foster the growth CAR T-cell therapy for multiple myeloma market globally. The presence of potential pipeline drugs to treat multiple myeloma is expected to boost the growth of the market in the coming years. However, high costs associated with CAR T-cell therapy owing to the high cost of research and development activities may hamper the growth of the market substantially over the estimated time frame.
The global CAR-T cell therapy for multiple myeloma market is segmented based on therapy type, and country. Based on the therapy type, the CAR T-cell therapy for multiple myeloma market is segmented into JNJ-68284528 (LCAR-B38M), Bb2121, CAR-CD44V6, P-BCMA-101, and others. Currently, there is no approved CAR- T cell therapy for multiple myeloma in the market. The presence of strong candidates under development stages is expected to accelerate the growth of the market in the coming years. Additionally, various research firms are trying combination therapy to treat multiple myeloma. The recent development in the understanding of immunology has permitted companies to develop new immunotherapeutic methods, that are effective in various cancers, including multiple myeloma. Based on the country, the market is studied across The US, Germany, France, the UK, Italy, and the Rest of the World.
|
Report Attribute |
Specifications |
|
Growth Rate CAGR |
CAGR of 27.0 % from 2023 to 2031 |
|
Quantitative Units |
Representation of revenue in US$ Million and CAGR from 2023 to 2031 |
|
Historic Year |
2019 to 2022 |
|
Forecast Year |
2023-2031 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Therapy Type |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
|
Competitive Landscape |
Bluebird bio/Celgene, Poseida Therapeutics, MolMed S.p.A., Janssen Research & Development, Celgene Corporation, CARsgen Therapeutics, Cartesian Therapeutics, and Precision BioSciences. |
|
Customization Scope |
Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing And Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global CAR T-Cell Therapy for Multiple Myeloma - Market Snapshot
Chapter 4. Global CAR T-Cell Therapy for Multiple Myeloma - Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Clinical Trial/Pipeline Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. Competitive Landscape & Market Share Analysis
4.8. Technology Advancement/Application Trends in CAR-T Cell Therapy
4.9. Incidence of Multiple Myeloma by Key Countries
4.10. Major Investment, Partnerships and Collaborations
4.11. Impact of COVID 19 on Immuno-oncology Industry
Chapter 5. Market Segmentation 1: Therapy Type Estimates & Trend Analysis
5.1. Therapy Type & Market Share, 2020 & 2030
5.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2018 to 2027 for the following Therapy Type:
5.2.1. Bb2121
5.2.2. JNJ-68284528 (LCAR-B38M)
5.2.3. P-BCMA-101
5.2.4. CAR-CD44V6
5.2.5. Others
Chapter 6. CAR T-Cell Therapy for Multiple Myeloma - Market Segmentation 3: Country/Region Estimates & Trend Analysis
6.1. The US
6.1.1. The US CAR T-Cell Therapy for Multiple Myeloma - Market revenue (US$ Million) estimates and forecasts by therapy type, 2020-2030
6.2. Germany
6.2.1. Germany CAR T-Cell Therapy for Multiple Myeloma - Market revenue (US$ Million) by therapy type, 2020-2030
6.3. France
6.3.1. France CAR T-Cell Therapy for Multiple Myeloma - Market revenue (US$ Million) by therapy type, 2020-2030
6.4. The UK
6.4.1. The UK CAR T-Cell Therapy for Multiple Myeloma - Market revenue (US$ Million) by therapy type, (US$ Million) 2020-2030
6.5. Italy
6.5.1. Italy revenue CAR T-Cell Therapy for Multiple Myeloma - Market revenue (US$ Million) by therapy type, (US$ Million) 2020-2030
6.6. Rest of World
6.6.1. Rest of World revenue CAR T-Cell Therapy for Multiple Myeloma - Market revenue (US$ Million) by therapy type, (US$ Million) 2020-2030
Chapter 7. Competitive Landscape
7.1. Major Mergers and Acquisitions/Strategic Alliances
7.2. Company Profiles
7.2.1. Bluebird bio/Celgene
7.2.2. Janssen Research & Development
7.2.3. Poseida Therapeutics
7.2.4. MolMed S.p.A.
7.2.5. Celgene Corporation
7.2.6. Cartesian Therapeutics
7.2.7. CARsgen Therapeutics
7.2.8. Precision BioSciences
Global CAR-T Cell Therapy for Multiple Myeloma Market by Therapy Type
Global CAR-T Cell Therapy for Multiple Myeloma Market Based on Country
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
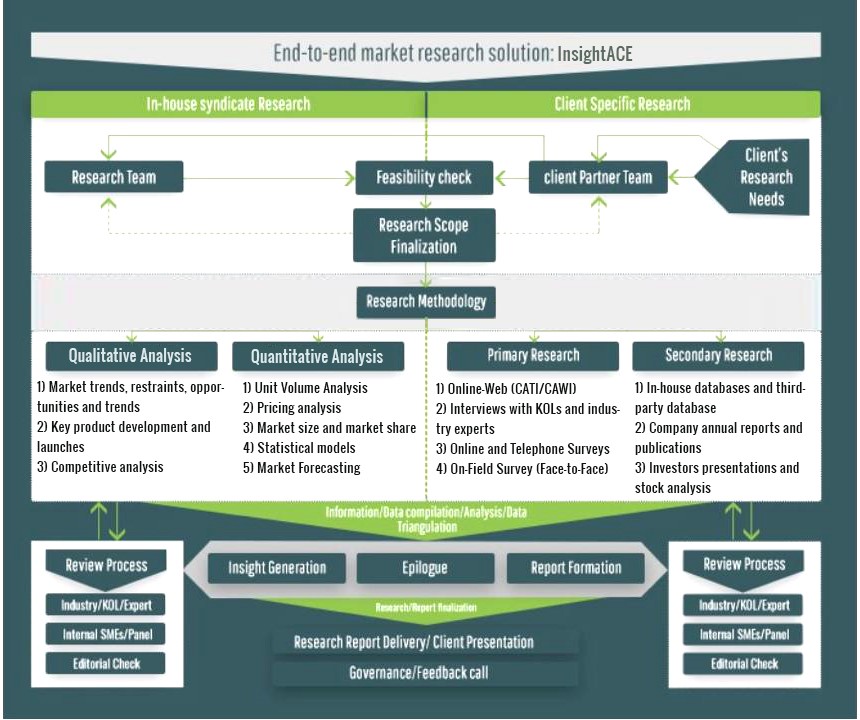
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.