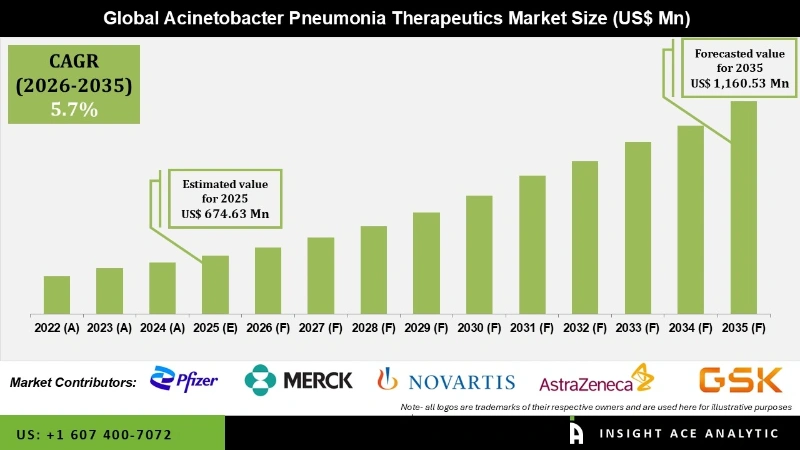
Global Acinetobacter Pneumonia Therapeutics Market Size is valued at USD 674.63 Mn in 2025 and is predicted to reach USD 1,160.53 Mn by the year 2035 at a 5.70% CAGR during the forecast period for 2026 to 2035.
Acinetobacter Pneumonia Therapeutics Market Size, Share & Trends Analysis Report By Drug Class (Cephalosporins, Carbapenems, Aminoglycosides, Polymyxins), By Route of Administration (Oral, Intravenous, Others), Region And Segment Forecasts, 2026 to 2035.
Acinetobacter pneumonia is a respiratory infection caused by bacteria from the Acinetobacter genus, namely Acinetobacter baumannii. These bacteria are opportunistic infections that are frequently associated with healthcare environments, posing a severe threat to those with weakened immune systems. Acinetobacter pneumonia causes fever, difficulty breathing, and coughing. The illness is difficult to treat due to increased antibiotic resistance in Acinetobacter strains. Prevention and control measures, such as stringent hygiene procedures in healthcare institutions, are critical to controlling the spread of Acinetobacter pneumonia, and continuing research is aimed at developing effective therapies for this persistent bacterial illness.
The market is being driven by the increasing prevalence of drug-resistant Acinetobacter strains, which needs the discovery of novel and effective treatments. The desire for innovative therapeutic solutions fuels the pharmaceutical industry's research and development operations. Aldo, Acinetobacter pneumonia is widely treated with a variety of antibiotic classes, including carbapenems, cephalosporins, aminoglycosides, and polymyxins. However, the evolution of resistance to these antibiotics presents a considerable concern.
The Acinetobacter Pneumonia Therapeutics Market is categorized on the basis of drug class and route of administration. The drug class segment includes Cephalosporins, Carbapenems, Aminoglycosides, and Polymyxins. The route of administration segment includes Oral, Intravenous, and Others.
Carbapenems are the leading product category in the Acinetobacter pneumonia treatments market. A type of drug that works very well against Acinetobacter infections is called carbapenems. They are frequently regarded as the first-line treatment for severe cases of Acinetobacter pneumonia due to their broad-spectrum action. Carbapenems are the medicine of choice for the therapeutic therapy of this illness since they have demonstrated great success in treating drug-resistant strains of Acinetobacter. Their widespread use and efficacy add to their market domination for Acinetobacter pneumonia treatments.
The Acinetobacter pneumonia treatments market's intravenous (IV) sector is increasing at the quickest rate. IV administration allows for the direct delivery of drugs into the bloodstream, resulting in rapid and efficient absorption. In the event of Acinetobacter pneumonia, where immediate treatment is critical, IV therapy is a fast and effective means to give antibiotics straight to the patient's system. This method of delivery is preferred in severe instances and for people who cannot take oral drugs. The rising use of IV treatments to treat Acinetobacter pneumonia contributes to the market segment's quick expansion.
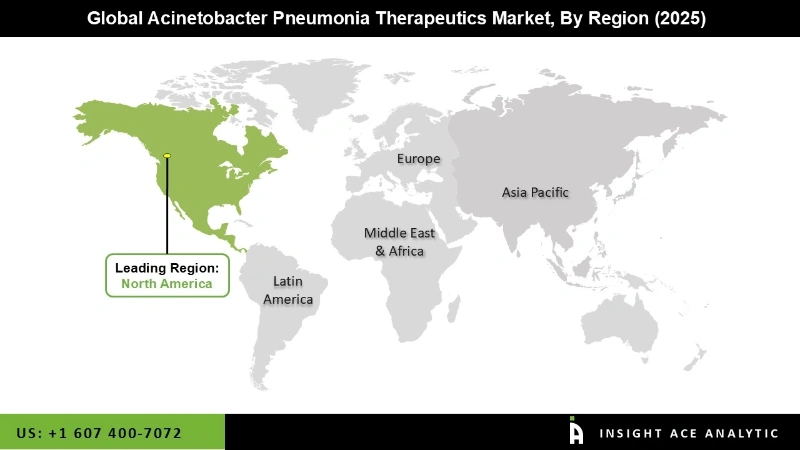
North America is predicted to be the largest market for acinetobacter pneumonia treatments throughout the forecast period. North America has long dominated the Acinetobacter pneumonia treatments market, thanks to the region's strong presence of top pharmaceutical companies and modern healthcare infrastructure. Countries such as the United States and Canada have seen a significant prevalence of pneumonia caused by Acinetobacter germs. Furthermore, Asia Pacific is predicted to be the second-largest market for acinetobacter pneumonia treatments in 2022, with a market share of more than 30.1%. Asia Pacific has recently emerged as the fastest-expanding market for Acinetobacter pneumonia treatments.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 674.63 Mn |
| Revenue Forecast In 2035 | USD 1,160.53 Mn |
| Growth Rate CAGR | CAGR of 5.70% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Drug Class, Route of Administration |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; France; Italy; Spain; Southeast Asia; South Korea |
| Competitive Landscape | Pfizer Inc., Merck & Co., Inc., GlaxoSmithKline plc (GSK), AstraZeneca plc, Novartis AG, Johnson & Johnson, Sanofi S.A., Bayer AG, and Basilea Pharmaceutical. |
| Customization Scope | Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Acinetobacter Pneumonia Therapeutics Market By Drug Class -
Acinetobacter Pneumonia Therapeutics Market By Route of Administration-
Acinetobacter Pneumonia Therapeutics Market By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.