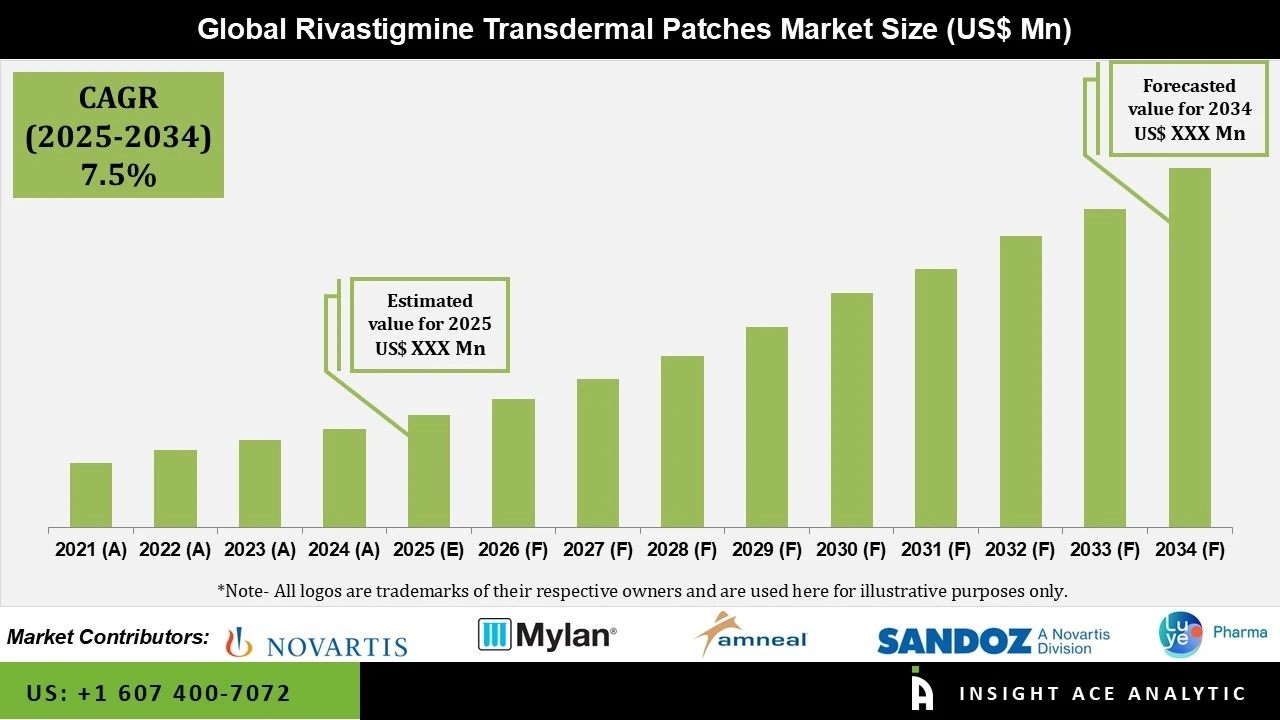
Global Rivastigmine Transdermal Patches Market Size is predicted to grow at a 7.5% CAGR during the forecast period for 2025-2034.
Rivastigmine transdermal patches are very effective in treating conditions where daily living is impacted by memory loss and cognitive decline. These patches allow patients to obtain medication with less gastrointestinal discomfort, which has frequently been linked to oral options, by using a controlled launch technique.
This transport technique enhances adherence and supports a clinician-led capacity to promptly modify treatment programs based on the patient's tolerance and scientific advancements. The increasing acceptance of transdermal techniques makes it easier to place this treatment favorably within long-term care systems where patients' comfort, consistency, and balance are important concerns.
The rising incidence of Alzheimer's disease and other types of dementia is one of the important factors in the growth of the rivastigmine transdermal patches market expansion. About 35 million individuals worldwide suffer from Alzheimer's disease, and that number is expected to triple by 2030, according to the World Health Organization. Rivastigmine transdermal patches, which are renowned for being easier to use than oral alternatives, are among the effective therapeutic choices that are desperately needed in view of this increasing prevalence. Additionally, the innovation in medication administration methods that improve patient outcomes is another significant element boosting the rivastigmine transdermal patches market growth. These patches' increased skin tolerability and longer use periods without irritation are made possible by the development of cutting-edge adhesive technology.
In addition, strategic partnerships between pharmaceutical firms and academic institutions promote patch formulation innovation, filling unmet medical requirements and broadening the Rivastigmine transdermal patches market's reach. The rising healthcare costs and government programs to increase public awareness of neurological conditions support market growth and foster an atmosphere that is favorable to Rivastigmine transdermal patches. Additionally, by improving the therapeutic advantages of Rivastigmine, continuous developments in pharmaceutical technologies such as the creation of smart patches and innovative drug delivery systems continue to drive market expansion. However, obstacles, including rivastigmine side effects, high treatment costs, and the availability of substitute treatments, are probably going to limit the Rivastigmine transdermal patches market expansion over the forecast period.
Driver
Growing Aging Population Globally
One major factor driving the growth of the Rivastigmine transdermal patch market is the aging of the world's population. The number of senior people, who are more vulnerable to neurodegenerative illnesses like Parkinson's and Alzheimer's, is increasing as life expectancy rises. The number of people 65 and older is predicted by the UN to increase to 1.5 billion by 2050. The need for efficient treatments to control aging-related cognitive impairment is increased by this demographic transition. For the long-term treatment of these disorders, rivastigmine transdermal patches are becoming more and more popular since they provide a non-invasive and patient-friendly alternative. As the number of senior people rises, healthcare systems are focusing more on enhancing quality of life, which is encouraging the use of medications like rivastigmine transdermal patches and propelling market expansion in both developed and developing nations.
Restrain/Challenge
High Cost of Rivastigmine Transdermal Patches
The rivastigmine transdermal patches market is significantly constrained by the high cost of these patches. Transdermal administration systems frequently have greater manufacturing and production costs than conventional oral drugs, which is reflected in their retail prices. Patients may find it more difficult to receive care as a result, especially in underdeveloped nations with limited healthcare funding. Additionally, patients may choose less expensive oral alternatives as a result of the financial stress, which could result in lower adoption rates. The high cost of rivastigmine patches may discourage patients and healthcare systems from completely adopting this treatment option, thus limiting market growth as healthcare professionals strive to strike a compromise between cost considerations and effective therapy.
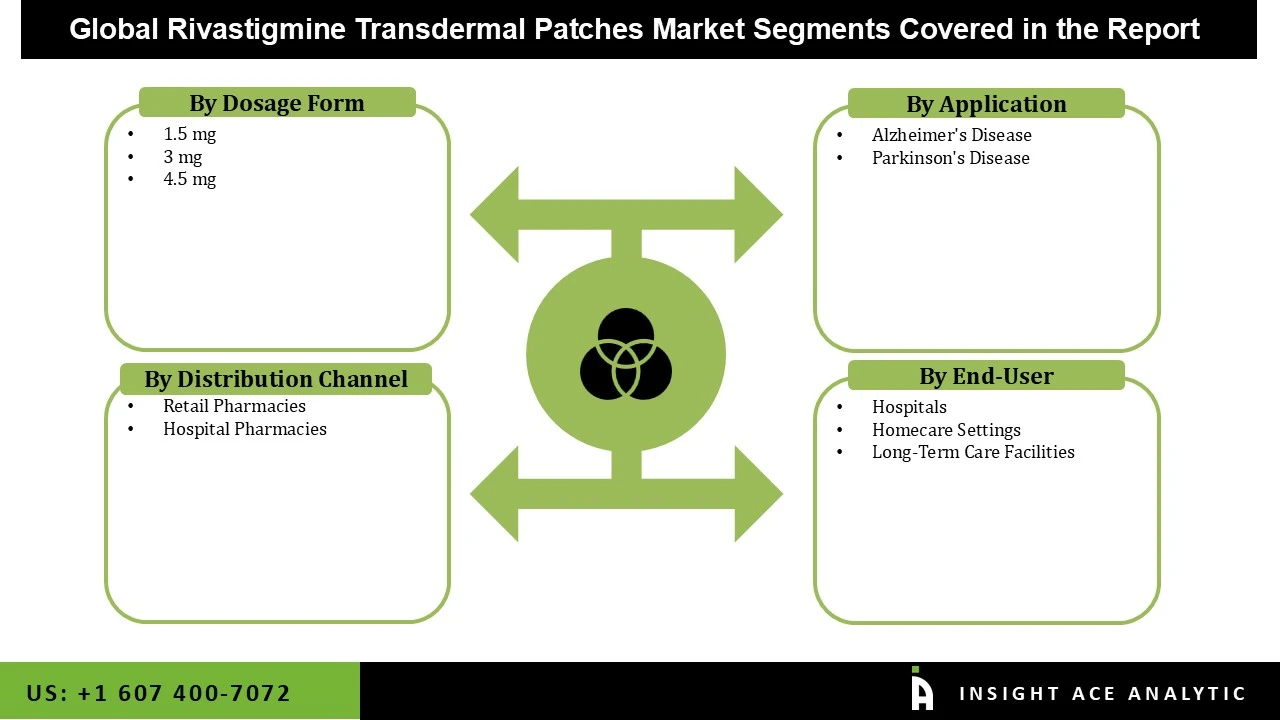
Rivastigmine transdermal patches market is categorized based on dosage form, application, end-user and distribution channel. Dosage form segment includes 1.5 mg patch, 3 mg patch, and 4.5 mg patch. Application is further segmented into alzheimer’s disease and parkinson’s disease. End-user is further segmented into hospitals, homecare settings, and long-term care facilities. Distribution channel is further segmented into hospital pharmacies and retail pharmacies.
The Alzheimer’s disease segment held the largest share in the rivastigmine transdermal patches market in 2024. The increasing demand for rivastigmine transdermal patches for the treatment of Alzheimer's disease is primarily due to the rising incidence of Alzheimer's disease worldwide, the growing preference for rivastigmine transdermal patches to administer medication to non-compliant patients, and patients experiencing side effects from oral drugs. Further boosting market acceptance include rising awareness of early-stage diagnosis, increased access to healthcare in emerging nations, and strong caregiver support for non-invasive treatment alternatives. The rivastigmine transdermal patch market's Alzheimer's disease category is growing faster overall due to ongoing advancements in patch design, dose accuracy, and skin tolerability.
In 2024, the hospitals segment dominated the rivastigmine transdermal patches market, driven by the growing number of patients with Parkinson's and Alzheimer's disease who need therapy to be started and monitored under supervision. Rivastigmine patches are frequently administered in hospitals for regulated dose titration, better patient compliance, and fewer gastrointestinal side effects than oral medications, all of which are important factors in the early detection of dementia-related illnesses. The demand is also being supported by an increase in older patient hospital admissions, the growth of neurology and geriatric care departments, and the increased accessibility of transdermal drug delivery methods.
The rivastigmine transdermal patchess market was dominated by the North America region in 2024 because the elderly population has a high incidence of dementias, including Alzheimer's disease. The region's sophisticated healthcare system and easy access to cutting-edge treatment alternatives are also driving market growth.
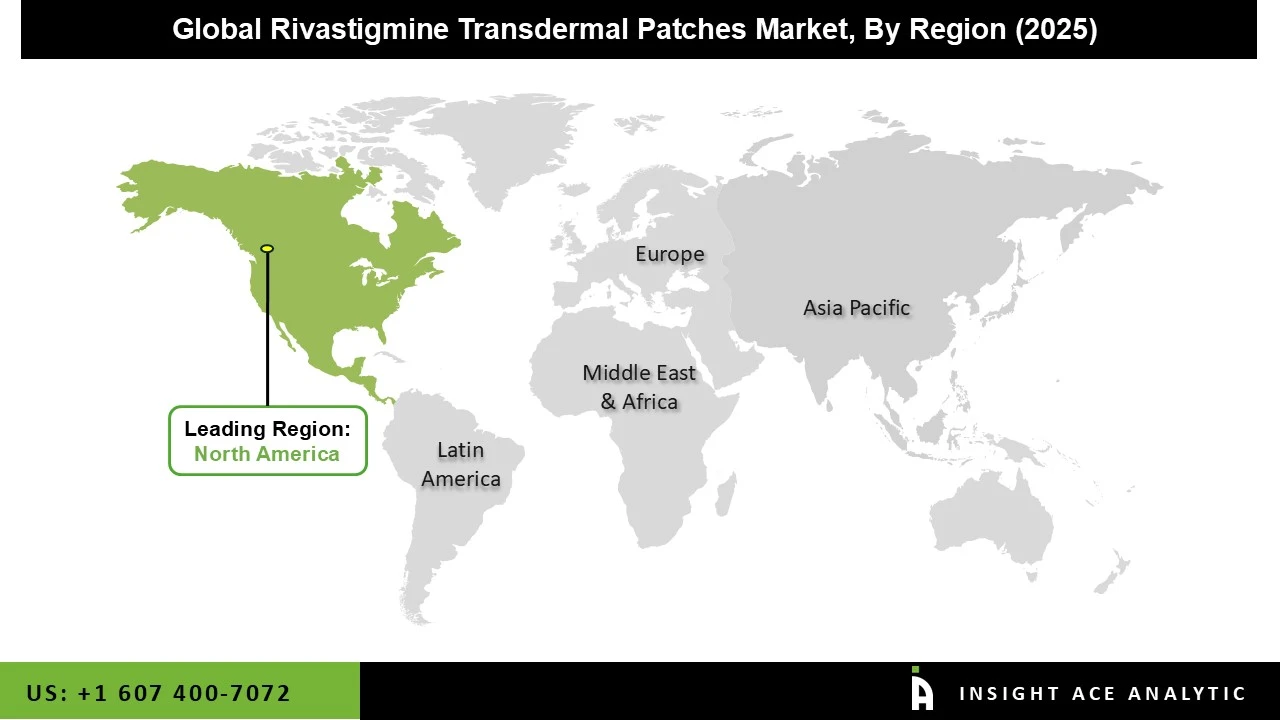
Furthermore, other factors that impact market dynamics in this region include rising governmental approvals, rapid technological developments in drug delivery systems, and partnerships between pharmaceutical corporations and academic institutions. Leading nations in the development and use of transdermal patches include the United States, where more than 6 million people suffer from Alzheimer's. Additionally, the presence of important companies and the increasing incidence of neurological illnesses are some of the reasons driving the market's expansion in the area.
October 2023: The National Medical Products Administration (NMPA) of China approved the Rivastigmine transdermal patch for Luye Pharma Group. The patches are widely used in Europe and be a promising treatment for symptomatic mild to severe Alzheimer's disease (AD).
| Report Attribute | Specifications |
| Growth Rate CAGR | CAGR of 7.5% from 2025 to 2034 |
| Quantitative Units | Representation of revenue in US$ Bn and CAGR from 2024 to 2034 |
| Historic Year | 2021 to 2023 |
| Forecast Year | 2024-2034 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Dosage Form, By Application, By End-user, By Distribution Channel, and By Region |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; Southeast Asia |
| Competitive Landscape | Bliss GVS Pharma, Novartis, Mylan Technologies (Viatris), Sandoz, Amneal Pharmaceuticals, Breckenridge Pharmaceutical, Yichang Humanwell, Zydus Pharmaceuticals, Luye Pharma Group, and Sino Biopharm. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.