Global GLP-1 Peptide Synthesis CDMO Market Size is predicted to grow at a 12.7 % CAGR during the forecast period for 2025-2034.
GLP-1 peptide synthesis CDMOs specialize in GLP-1 peptide synthesis, offering services from research-grade to GMP-compliant production for pharmaceutical applications.
GLP-1 (Glucagon-Like Peptide-1) is a peptide hormone synthesized from the proglucagon gene in intestinal L-cells and certain neurons. It plays a crucial role in regulating blood sugar levels by stimulating insulin release and inhibiting glucagon secretion.
Advanced techniques such as solid-phase peptide synthesis (SPPS), solution-phase synthesis, or hybrid methods are used to produce GLP-1 peptides. These peptides can be manufactured at various scales, ranging from small research batches to large-scale commercial production, ensuring their availability for both scientific and therapeutic applications. Drug companies often prefer to outsource GLP-1 peptide synthesis to CDMOs due to the complexity and scalability challenges associated with this process as CDMOs offer specialized expertise, advanced infrastructure, and cost-effective solutions for both research and large-scale production of GLP-1 peptides.
Factors such as surging market demand for the global GLP-1 receptor agonists, therapeutic expansion of GLP-1 Beyond diabetes in obesity, cardiovascular diseases, and neurodegenerative disorders treatment, sophisticated synthesis requirements for GLP-1 peptides (e.g., solid-phase peptide synthesis or hybrid approaches), high-purity standards, and impurity control, shortages of high-quality raw materials and the need for cost-effective, sustainable manufacturing and stringent FDA and EMA requirements for GMP-grade peptides fuel the need for scalable, high-quality peptide production and drug manufacturing companies to outsource manufacturing to the GLP-1 peptide synthesis CDMOs.
The growing global prevalence of type 2 diabetes and obesity has significantly increased demand for GLP-1 receptor agonists such as semaglutide, liraglutide, and tirzepatide, which are known for their ability to manage blood glucose levels, support weight loss, and improve cardiovascular outcomes. This surge in demand is driving the need for large-scale peptide production, making CDMOs essential partners in this process. As peptides gain popularity for their high selectivity, potency, and favorable safety profiles, GLP-1 analogues have emerged as a key segment within the peptide therapeutics market, encouraging pharmaceutical companies to outsource their production to specialized CDMOs increasingly.
The GLP-1 peptide synthesis CDMO market is segmented based on synthesis technology, service type, scale of operation, and end-user. Based on synthesis technology, the market is segmented into solid-phase peptide synthesis (SPPS), liquid-phase peptide synthesis (LPPS), group-assisted purification peptide synthesis (GAP-PS), hybrid technology, and recombinant DNA technology. Based on the type of service, the market is divided into analytical and process development, large-scale production, custom peptide synthesis, peptide modification, purification technology, formulation development, regulatory support, packaging and labeling, pre-formulation, and registration batches. Based on the scale of operation, the market is divided into clinical-scale manufacturing, commercial-scale manufacturing, and pilot-scale manufacturing. Based on the end-user, the market is divided into pharmaceutical companies, biotechnology companies, and research institutions.
The solid-phase peptide synthesis (SPPS) segment is expected to have the highest growth rate during the forecast period. GLP-1 analogues, such as semaglutide and other long-chain, structurally complex peptides, are best synthesised via solid-phase peptide synthesis (SPPS). Compared to solution-phase techniques, it permits the gradual addition of amino acids, providing for more control and oversight. To improve the stability, bioactivity, and pharmacokinetics of GLP-1 analogues, SPPS also supports the addition of non-natural amino acids, PEGylation, lipidation, and other chemical modifications. Due to these qualities, SPPS is a crucial platform for the creation and manufacture of future-generation GLP-1 receptor agonists.
The large-scale production segment is expected to dominate the market. There is a demand for kilogram-scale, GMP-compliant peptide manufacturing, as many GLP-1 analogues have progressed from clinical development to full commercialization. Large-scale CDMOs are crucial for providing reliable, superior, and legally compliant manufacturing at industrial scales. The cost-per-gram of peptide active pharmaceutical ingredients (APIs) is significantly decreased by economies of scale made possible by large-scale manufacture. Pharmaceutical businesses seeking dependable and efficient manufacturing partners find CDMOs with automated solid-phase peptide synthesis (SPPS) systems and optimized batch processes to be highly appealing, as they can offer competitive pricing and expedited turnaround times.
North America, particularly the US, is one of the largest markets for GLP-1 receptor agonists such as Ozempic, Wegovy, and Mounjaro. The demand for GLP-1 analogue manufacture is greatly influenced by the fact that the US leads the world in pharmaceutical research and development, particularly in the fields of diabetes and obesity treatments. The region's CDMOs are favored partners for the clinical and commercial-scale manufacturing of GLP-1 peptides due to their sophisticated infrastructure and strict adherence to FDA standards. The market in North America is expected to increase due to its sophisticated manufacturing capabilities, robust pharmaceutical demand, and concentration of top-tier CDMOs that can support large-scale clinical and commercial peptide production.
|
Report Attribute |
Specifications |
|
Growth Rate CAGR |
CAGR of 12.7 % from 2025 to 2034 |
|
Quantitative Units |
Representation of revenue in US$ Mn and CAGR from 2025 to 2034 |
|
Historic Year |
2021 to 2024 |
|
Forecast Year |
2025-2034 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Synthesis Technology, By Service Type, By Scale of Operation, By End-User |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; South East Asia |
|
Competitive Landscape |
Bachem Holding AG, CordenPharma, PolyPeptide Group, AmbioPharm, CPC Scientific, CSBio, Creative Peptides, Lonza, Aurisco Pharmaceutical, Hybio Pharmaceuticals, Chinese Peptide Company, Neuland Laboratories, Divis Laboratories, Supriya Lifescience, Allsino Pharmaceutical Co. Ltd |
|
Customization Scope |
Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing and Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global GLP-1 Peptide Synthesis CDMO Market Snapshot
Chapter 4. Global GLP-1 Peptide Synthesis CDMO Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Porter's Five Forces Analysis
4.7. Incremental Opportunity Analysis (US$ MN), 2024-2034
4.8. Competitive Landscape & Market Share Analysis, By Key Player (2024)
4.9. Use/impact of AI on GLP-1 Peptide Synthesis CDMO Market Industry Trends
4.10. Global GLP-1 Peptide Synthesis CDMO Market Penetration & Growth Prospect Mapping (US$ Mn), 2021-2034
Chapter 5. GLP-1 Peptide Synthesis CDMO Market Segmentation 1: By Synthesis Technology, Estimates & Trend Analysis
5.1. Market Share by Synthesis Technology, 2024 & 2034
5.2. Market Size Revenue (US$ Million) & Forecasts and Trend Analyses, 2021 to 2034 for the following Synthesis Technology:
5.2.1. Solid-Phase Peptide Synthesis (SPPS)
5.2.2. Liquid-Phase Peptide Synthesis (LPPS)
5.2.3. Group-Assisted Purification Peptide Synthesis (GAP-PS)
5.2.4. Hybrid Service Type
5.2.5. Recombinant DNA Service Type
Chapter 6. GLP-1 Peptide Synthesis CDMO Market Segmentation 2: By Service Type, Estimates & Trend Analysis
6.1. Market Share by Service Type, 2024 & 2034
6.2. Market Size Revenue (US$ Million) & Forecasts and Trend Analyses, 2021 to 2034 for the following Service Type:
6.2.1. Analytical and Process Development
6.2.2. Large-Scale Production
6.2.3. Custom Peptide Synthesis
6.2.4. Peptide Modification
6.2.5. Purification Technology
6.2.6. Formulation Development
6.2.7. Regulatory Support
6.2.8. Packaging and Labeling
6.2.9. Pre-Formulation and Registration Batches
Chapter 7. GLP-1 Peptide Synthesis CDMO Market Segmentation 3: By Scale of Operation, Estimates & Trend Analysis
7.1. Market Share by Scale of Operation, 2024 & 2034
7.2. Market Size Revenue (US$ Million) & Forecasts and Trend Analyses, 2021 to 2034 for the following Scale of Operation:
7.2.1. Clinical-Scale Manufacturing
7.2.2. Commercial-Scale Manufacturing
7.2.3. Pilot-Scale Manufacturing
Chapter 8. GLP-1 Peptide Synthesis CDMO Market Segmentation 4: By End User, Estimates & Trend Analysis
8.1. Market Share by End User, 2024 & 2034
8.2. Market Size Revenue (US$ Million) & Forecasts and Trend Analyses, 2021 to 2034 for the following End User:
8.2.1. Pharmaceutical Companies
8.2.2. Biotechnology Companies
8.2.3. Research Institutions
Chapter 9. GLP-1 Peptide Synthesis CDMO Market Segmentation 5: Regional Estimates & Trend Analysis
9.1. Global GLP-1 Peptide Synthesis CDMO Market, Regional Snapshot 2024 & 2034
9.2. North America
9.2.1. North America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Country, 2021-2034
9.2.1.1. US
9.2.1.2. Canada
9.2.2. North America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Synthesis Technology, 2021-2034
9.2.3. North America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Service Type, 2021-2034
9.2.4. North America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Scale of Operation, 2021-2034
9.2.5. North America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by End User, 2021-2034
9.3. Europe
9.3.1. Europe GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Country, 2021-2034
9.3.1.1. Germany
9.3.1.2. U.K.
9.3.1.3. France
9.3.1.4. Italy
9.3.1.5. Spain
9.3.1.6. Rest of Europe
9.3.2. Europe GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Synthesis Technology, 2021-2034
9.3.3. Europe GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Service Type, 2021-2034
9.3.4. Europe GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Scale of Operation, 2021-2034
9.3.5. Europe GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by End User, 2021-2034
9.4. Asia Pacific
9.4.1. Asia Pacific GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Country, 2021-2034
9.4.1.1. India
9.4.1.2. China
9.4.1.3. Japan
9.4.1.4. Australia
9.4.1.5. South Korea
9.4.1.6. Hong Kong
9.4.1.7. Southeast Asia
9.4.1.8. Rest of Asia Pacific
9.4.2. Asia Pacific GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Synthesis Technology, 2021-2034
9.4.3. Asia Pacific GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Service Type, 2021-2034
9.4.4. Asia Pacific GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Scale of Operation, 2021-2034
9.4.5. Asia Pacific GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by End User, 2021-2034
9.5. Latin America
9.5.1. Latin America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Country, 2021-2034
9.5.1.1. Brazil
9.5.1.2. Mexico
9.5.1.3. Rest of Latin America
9.5.2. Latin America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Synthesis Technology, 2021-2034
9.5.3. Latin America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Service Type, 2021-2034
9.5.4. Latin America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Scale of Operation, 2021-2034
9.5.5. Latin America GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by End User, 2021-2034
9.6. Middle East & Africa
9.6.1. Middle East & Africa Wind Turbine Rotor Blade Market Revenue (US$ Million) Estimates and Forecasts by country, 2021-2034
9.6.1.1. GCC Countries
9.6.1.2. Israel
9.6.1.3. South Africa
9.6.1.4. Rest of Middle East and Africa
9.6.2. Middle East & Africa GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Synthesis Technology, 2021-2034
9.6.3. Middle East & Africa GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Service Type, 2021-2034
9.6.4. Middle East & Africa GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by Scale of Operation, 2021-2034
9.6.5. Middle East & Africa GLP-1 Peptide Synthesis CDMO Market Revenue (US$ Million) Estimates and Forecasts by End User, 2021-2034
Chapter 10. Competitive Landscape
10.1. Major Mergers and Acquisitions/Strategic Alliances
10.2. Company Profiles
10.2.1. Bachem Holding AG
10.2.1.1. Business Overview
10.2.1.2. Key Synthesis Service Type/Service Overview
10.2.1.3. Financial Performance
10.2.1.4. Geographical Presence
10.2.1.5. Recent Developments with Business Strategy
10.2.2. CordenPharma
10.2.3. PolyPeptide Group
10.2.4. AmbioPharm
10.2.5. CPC Scientific
10.2.6. CSBio
10.2.7. Creative Peptides
10.2.8. Lonza
10.2.9. Aurisco Pharmaceutical
10.2.10. Hybio Pharmaceuticals
10.2.11. Chinese Peptide Company
10.2.12. Neuland Laboratories
10.2.13. Divis Laboratories
10.2.14. Supriya Lifescience
10.2.15. Allsino Pharmaceutical Co. Ltd
10.2.16. Other Prominent Players
Global GLP-1 Peptide Synthesis CDMO Market - By Synthesis Technology
Global GLP-1 Peptide Synthesis CDMO Market – By Service Type
Global GLP-1 Peptide Synthesis CDMO Market – By Scale of Operation
Global GLP-1 Peptide Synthesis CDMO Market – By End-User
Global GLP-1 Peptide Synthesis CDMO Market – By Region
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
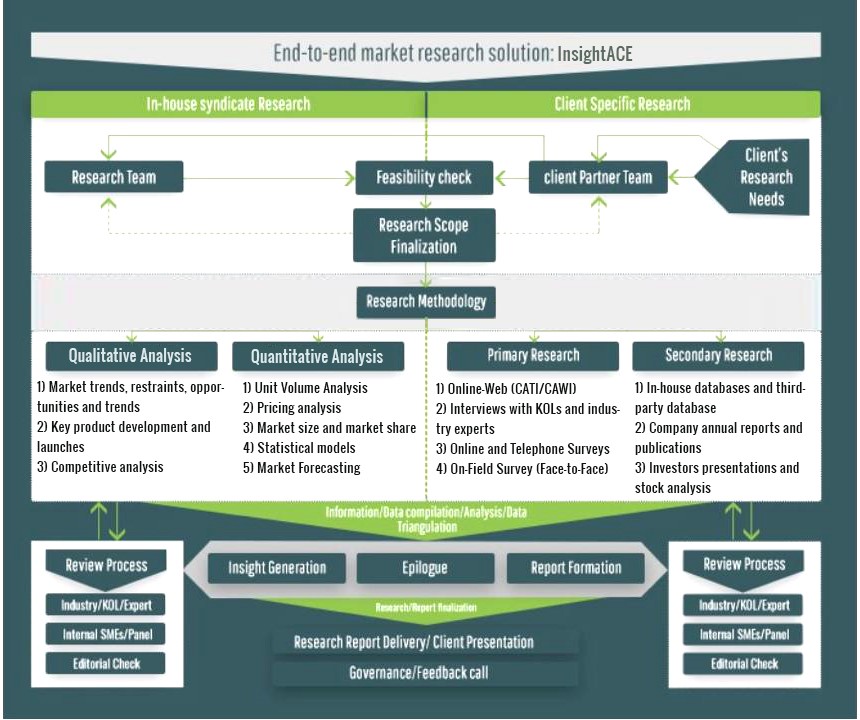
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.