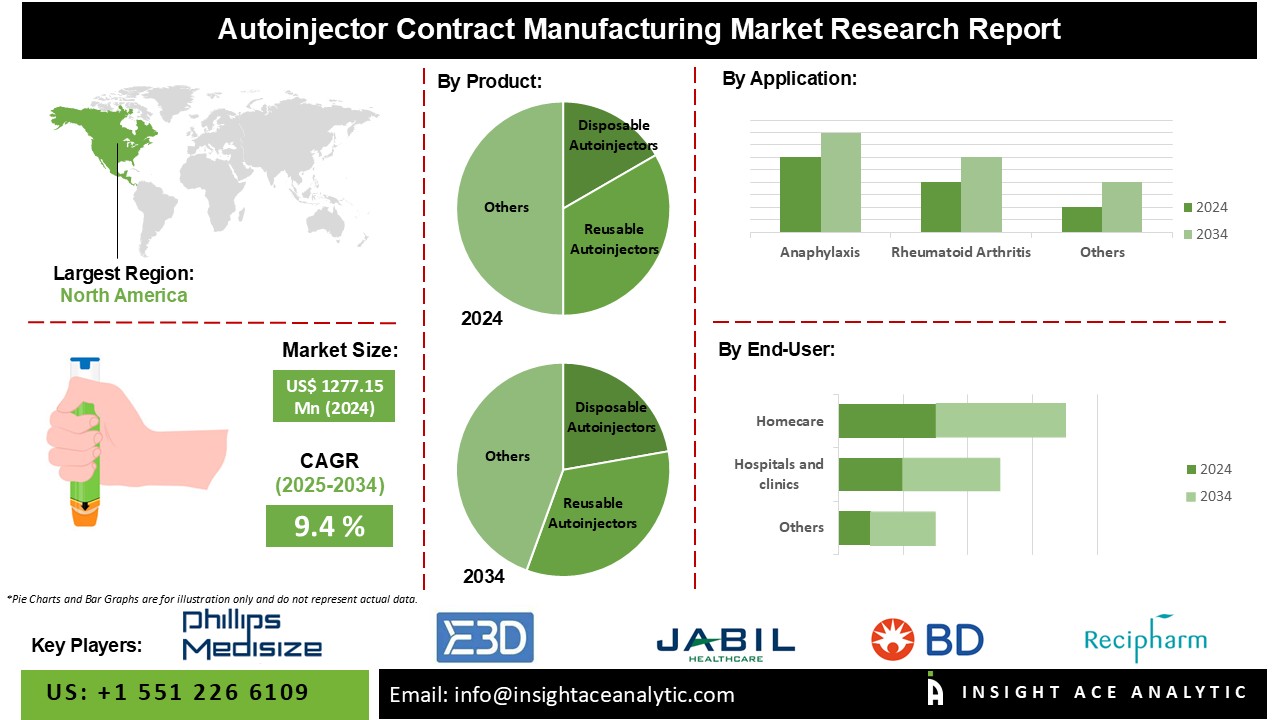
Autoinjector Contract Manufacturing Market Size is valued at 1277.15 Million in 2024 and is predicted to reach 3077.72 Million by the year 2034 at a 9.4% CAGR during the forecast period for 2025-2034.
Autoinjectors are self-injecting devices. They are a significant type of medical device that may administer medications subcutaneously or intramuscularly. They contain spring-driven prefilled syringes or cartridges. As medical equipment, autoinjectors primarily dispense drugs. They are typically utilized by healthcare experts due to their various advantages. An autoinjector is preferable to alternative methods because it maintains medicine dosage precision and lessens needle anxiety, among other benefits. The market for Autoinjector Contract Manufacturing is expanding as a result of rising anaphylaxis, targeted treatments, and regulatory approvals. The prevalence of disorders including diabetes, rheumatoid arthritis, multiple sclerosis, and others is increasing, which is what is driving the global market for auto-injectors. Age is a factor in rheumatoid arthritis risk, which peaks between the ages of 35 and 50. Therefore, it is projected that an increase in the elderly population will fuel the global market for autoinjector contract manufacturing.
The global market is anticipated to be driven by an increase in the number of injectable medications. In addition, the use of new technologies is a significant driver that is projected to boost the global market for autoinjector contract manufacturing. The ease of use, lower risk of infection, and mobility of auto-injectors have increased their use, which in turn has boosted the growth of the global market for these devices. The infrastructure for health care is being modernized by governments in emerging nations, which is expected to expand access to healthcare. This is anticipated to increase demand for cutting-edge technology, which would in turn provide attractive market prospects for autoinjector contract manufacturing around the world.
The growing popularity of alternate drug delivery techniques like nasal and oral medication delivery limits the market for Autoinjector Contract Manufacturing. Autoinjectors can reduce needle-related anxiety to some extent, but they cannot completely eliminate it. This is especially true for children and young people, which also acts as a barrier to the market's expansion for Autoinjector Contract Manufacturing.
The Autoinjector Contract Manufacturing Market is segmented on the basis of Product, Application, and End-User. Based on Product, the market is segmented as Disposable Autoinjectors, Reusable Autoinjectors, Wearable Injector, Smart/Connected Autoinjectors. Based on Application, the market is segmented as Anaphylaxis, Rheumatoid Arthritis, Multiple Sclerosis, Diabetes, and Others. Based on End-User, the market is segmented as Homecare, Hospitals & Clinics, and Ambulatory Services.
Based on application, the rheumatoid arthritis segment is accounted as a major contributor to the Autoinjector Contract Manufacturing Market
The market for rheumatoid arthritis autoinjectors accounted for the majority of sales. Autoinjectors enable patients with rheumatoid arthritis to self-administer their medications because they are frequently unable to inject their medications. The rheumatoid arthritis sector is anticipated to maintain its commanding market position given the rising geriatric population and the increasing number of rheumatoid arthritis cases over the world. The primary drivers driving the growth of the segment are the entry of biosimilar drugs, a rise in the elderly population, an increase in the use of conventional DMARDs, and government initiatives to disseminate awareness of rheumatoid arthritis symptoms. Other reasons that contribute to the segment expansion include advancements in advanced biologics, an increase in healthcare spending, increased purchasing power, and access to high-quality medications for poor and middle-class families worldwide.
Based on End-User, the home care segment is accounted as a major contributor to the Autoinjector Contract Manufacturing Market
The market for Autoinjector Contract Manufacturing was dominated by the home care segment. Autoinjector Contract Manufacturing eliminate the need for specialized medical care when giving medication to elderly individuals who frequently need periodic injections of their medications. Additionally enabling patients to self-administer medication and lowering the cost per injection are Autoinjector Contract Manufacturing. The two main factors driving the homecare market are the aging population and the demand for less expensive treatments. The market for home care services is being supported by the existence of numerous organizations providing these services in North America and Europe. The category is therefore predicted to grow during the forecast period due to an increase in the elderly population and nursing home care.
In the region, the North America Autoinjector Contract Manufacturing Market holds a significant revenue share.
North America is expected to have a significant market share for autoinjector contract manufacturing over the projection period due to the early acceptance of technologically sophisticated goods and product approvals. The high diabetes population and rising healthcare infrastructure, which have attracted several prominent autoinjector device manufacturers to this region, are the main drivers of market growth in the region. Through various strategies, including opening sales offices and collaborating with regional pharmaceutical firms, these businesses are expanding their footprint in the regional market. The rise in incidences of anaphylaxis, rheumatoid arthritis, and other illnesses like anemia and migraine, as well as continued corporate technical developments, are predicted to boost the market's overall expansion.
| Report Attribute | Specifications |
| Market Size Value In 2024 | USD 1277.15 Million |
| Revenue Forecast In 2034 | USD 3077.72 Million |
| Growth Rate CAGR | CAGR of 9.4% from 2025 to 2034 |
| Quantitative Units | Representation of revenue in US$ Million, Volume (Unit), and CAGR from 2025 to 2034 |
| Historic Year | 2021 to 2024 |
| Forecast Year | 2025-2034 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Product, By Application, By End-User |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Phillips-Medisize (a Molex Company), Elcam Drug Delivery Devices (E3D) (Elcam Medical), Jabil Healthcare, Dali Medical Devices, Ypsomed Delivery Systems (YDS), SHL Medical, Haselmeier, Owen Mumford, Becton, Dickinson and Company (BD), CCBio, Nemera (Copernicus), Recipharm AB, Sonceboz, Solteam Medical, Stevanato Group, Union Medico ApS., Neuma Engineering, Emperra, Eitan Medical, Enable Injections, West Pharmaceutical Services, Inc., Delfu Medical, Wanhai Medical, and Other Prominent Player. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
By Product
By Application
By End-User
By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.