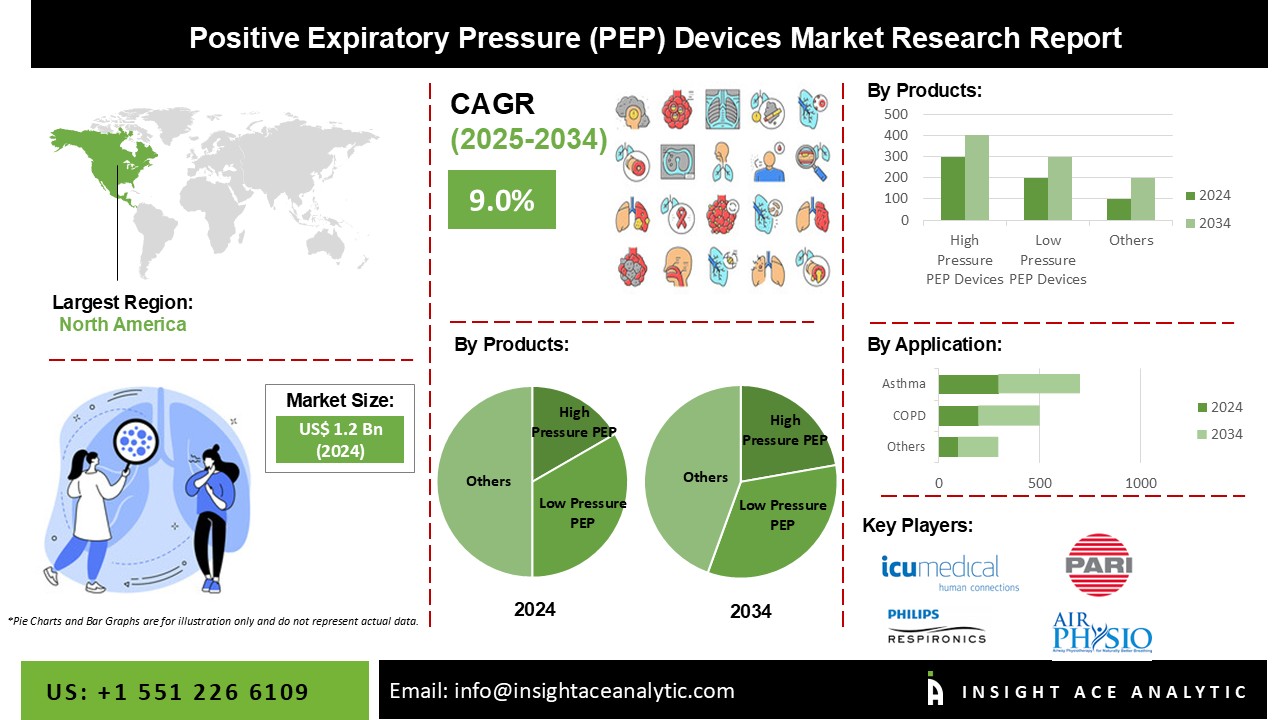
Positive Expiratory Pressure (PEP) Devices Market Size is valued at 1.2 Billion in 2024 and is predicted to reach 2.8 Billion by the year 2034 at a 9.0% CAGR during the forecast period for 2025-2034.
Positive expiratory pressure therapy (PEP) is performed for airway clearance therapy. A participant in this therapy breathes through a portable mouthpiece or mask. This allows air to pass past the mucus in the lungs and away from the airway walls. The device facilitates unrestricted air passage and requires active inhaled air expiration against resistance. The growth in COPD prevalence due to increased smoking and drinking in industrialized countries, as well as the recent introductions of innovative oscillatory PEP, are pushing the positive expiratory pressure devices market. The rising frequency of chronic pulmonary disorders and technological developments drive market expansion. The PEP devices market is growing due to an ageing population and an increase in chronic disease occurrences.
However, in low-income countries, a lack of awareness regarding technologically advanced airway clearing medicines is projected to stymie the worldwide Positive Expiratory Pressure Devices market growth. Furthermore, the market is expected to advance due to the time-consuming old airway clearance techniques.
The Positive Expiratory Pressure (PEP) Devices market is segmented on the basis of Product Type, Application. By product, the market is segmented as High Pressure PEP (26-102cm H2O) Devices, and Low Pressure PEP (5-20cm H2O) Devices. The Application segment includes Chronic Obstructive Pulmonary Disease (COPD), Asthma, Atelectasis, Bronchitis, Bronchiectasis, Cystic Fibrosis, and Others.
The global PEP devices market can be divided into two product types: high-pressure PEP (26-102cm H2O) and low-pressure PEP (5-20cm H2O). The high-pressure PEP category earned the most revenue, owing to increased patient compliance with high-pressure devices and increased bronchodilation achieved by pressures more significant than 26 cm H2O. The enhanced patient initiatives to shorten hospital stays are expected to grow the high-pressure PEP segment. PEP devices with high pressure are used to give high levels of positive expiratory pressure (PEP) to the lungs. These devices are commonly utilized in individuals suffering from the chronic obstructive pulmonary disease (COPD), asthma, or other respiratory disorders requiring improved airway clearance. High-pressure PEP devices can be mechanical or electrical, and they can generate the appropriate level of PEP through various processes. Some high-pressure PEPs can be modified to meet the needs of the patient's breathing by turning a knob on the device known as an expiratory resistance control valve, or ERV.
The market will be led by chronic obstructive pulmonary disease (COPD). COPD is caused by an obstruction in the airways, causing difficulty breathing, and is primarily caused by tobacco smoking. According to a 2019 WHO (World Health Organization) report, the chronic obstructive pulmonary disease is the third leading cause of death worldwide. Growing patient awareness for the creation of a shared platform for COPD sufferers is likely to boost the segment's growth during the forecast period.
North America dominates the positive expiratory pressure (PEP) market because of the increasing patient awareness in the region. Some of the factors driving the oscillatory positive expiratory pressure (OPEP) market during the forecast period include the rising prevalence of chronic obstructive pulmonary disease (COPD), increasing technological advancements, rising prevalence of asthma, and continuous technological and research development. The increasing frequency of chronic illnesses, on the other hand, will create several opportunities, resulting in the rise of the positive expiratory pressure (PEP) market within the time period stated. Due to an expanding patient pool and raised spending on healthcare infrastructure development by developing countries, the Asia-Pacific region is projected to expand during the projected period.
|
Report Attribute |
Specifications |
|
Market Size Value In 2024 |
USD 1.2 Billion |
|
Revenue Forecast In 2034 |
USD 2.8 Billion |
|
Growth Rate CAGR |
CAGR of 9.0 % from 2025 to 2034 |
|
Quantitative Units |
Representation of revenue in US$ Billion and CAGR from 2025 to 2034 |
|
Historic Year |
2021 to 2024 |
|
Forecast Year |
2025-2034 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Product Type, By Application |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; South Korea; South East Asia |
|
Competitive Landscape |
Smiths Medical, Inc. (ICU Medical), Philips Respironics (Koninklijke Philips N.V.), Monaghan Medical Corporation, D-R Burton Healthcare, PARI GmbH, AirPhysio, R. Cegla GmbH & Co. KG, Trudell Medical International, Allergan Plc. (AbbVie), Aptalis Pharma US, Inc. (Nestlé Health SoloPep, Medica Holdings, LLC,Intersurgical Ltd.,Clement Clarke International (Haag-Streit Group), Hill-Rom Holdings (Baxter International) |
|
Customization Scope |
Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing And Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global Positive Expiratory Pressure (PEP) Devices Market Snapshot
Chapter 4. Global Positive Expiratory Pressure (PEP) Devices Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Market Dynamics - Drivers
4.3. Challenges/Restraints
4.4. Market Trends
4.5. Industry Analysis – Porter’s Five Forces Analysis
4.6. Competitive Landscape & Market Share Analysis
Chapter 5. Market Segmentation 1: Product Type Estimates & Trend Analysis
5.1. Product Type & Market Share, 2024 & 2034
5.2. Market Size (Value & Volume) & Forecasts and Trend Analyses, 2021 & 2034 for the following Product Type:
5.2.1. High Pressure PEP (26-102cm H2O) Devices
5.2.2. Low Pressure PEP (5-20cm H2O) Devices
Chapter 6. Positive Expiratory Pressure (PEP) Devices Market Segmentation 2: Application Estimates & Trend Analysis
6.1. Application Analysis & Market Share, 2024 & 2034
6.2. Market Size (Value & Volume) & Forecasts and Trend Analyses, 2021 & 2034 for the following Application:
6.2.1. Chronic Obstructive Pulmonary Disease (COPD)
6.2.2. Asthma
6.2.3. Atelectasis
6.2.4. Bronchitis
6.2.5. Bronchiectasis
6.2.6. Cystic Fibrosis
6.2.7. Others
Chapter 7. Positive Expiratory Pressure (PEP) Devices Market Segmentation 3: Regional Estimates & Trend Analysis
7.1. North America
7.1.1. North America Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Product Type, 2021 & 2034
7.1.2. North America Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Application, 2021 & 2034
7.1.3. North America Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by country, 2021 & 2034
7.1.3.1. U.S.
7.1.3.2. Canada
7.2. Europe
7.2.1. Europe Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Product Type, 2021 & 2034
7.2.2. Europe Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Application, 2021 & 2034
7.2.3. Europe Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) by country, 2021 & 2034
7.2.3.1. Germany
7.2.3.2. Poland
7.2.3.3. France
7.2.3.4. Italy
7.2.3.5. Spain
7.2.3.6. UK
7.2.3.7. Rest of Europe
7.3. Asia Pacific
7.3.1. Asia Pacific Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Product Type, 2021 & 2034
7.3.2. Asia Pacific Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Application, 2021 & 2034
7.3.3. Asia Pacific Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) by country, 2021 & 2034
7.3.3.1. China
7.3.3.2. India
7.3.3.3. Japan
7.3.3.4. Australia
7.3.3.5. Rest of Asia Pacific
7.4. Latin America
7.4.1. Latin America Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Product Type, 2021 & 2034
7.4.2. Latin America Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Application, 2021 & 2034
7.4.3. Latin America Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) by country, (US$ Million) 2021 & 2034
7.4.3.1. Brazil
7.4.3.2. Rest of Latin America
7.5. MEA
7.5.1. MEA Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Product Type, 2021 & 2034
7.5.2. MEA Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) estimates and forecasts by Application, 2021 & 2034
7.5.3. MEA revenue Positive Expiratory Pressure (PEP) Devices Market revenue (US$ Million) and volume (No. of Units) by country, (US$ Million) 2021 & 2034
7.5.3.1. South Africa
7.5.3.2. Rest of MEA
Chapter 8. Competitive Landscape
8.1. Major Mergers and Acquisitions/Strategic Alliances
8.2. Company Profiles
8.2.1. Smiths Medical (ICU Medical)
8.2.1.1. D-R Burton Healthcare
8.2.1.2. PARI GmbH
8.2.1.3. AirPhysio
8.2.1.4. R. Cegla GmbH & Co. KG
8.2.2. Trudell Medical International
8.2.3. Allergan Plc. (AbbVie)
8.2.4. Aptalis Pharma US, Inc. (Nestlé Health Science)
8.2.5. SoloPep
8.2.6. Medica Holdings, LLC
8.2.7. Intersurgical Ltd.
8.2.8. Clement Clarke International (Haag-Streit Group)
8.2.9. Hill-Rom Holdings (Baxter International)
8.2.8. Monaghan Medical Corporation
8.2.9. Philips Respironics (Koninklijke Philips N.V.)
8.3. List of Other Prominent Players
By Product Type
By Application
By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
Rest of Middle East and Africa
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.