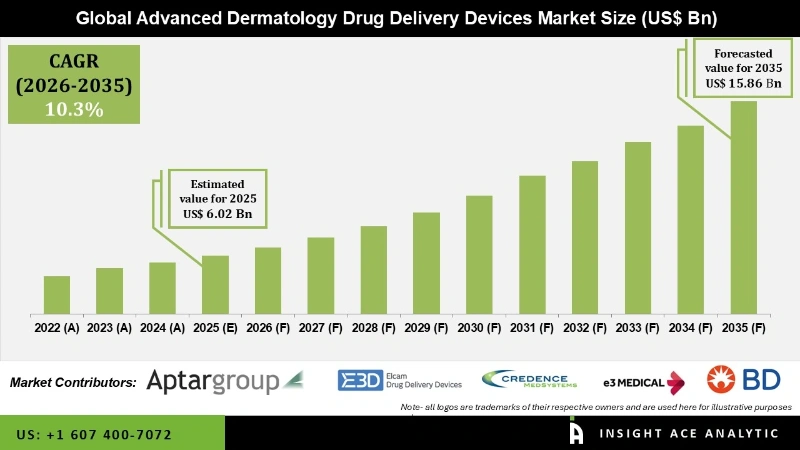
Advanced Dermatology Drug Delivery Devices Market Size is valued at 6.02 Billion in 2025 and is predicted to reach 15.86 Billion by 2035 at a 10.30% CAGR during the forecast period for 2026 to 2035.
Advanced Dermatology Drug Delivery Devices Market Size, Share & Trends Analysis Report By Product (Injectors, Patches, Dermal Pumps, Airless Dispensers and Bottles, Valves, Other Devices), By Sales Channel, By End-Users, By Region, And By Segment Forecasts, 2026 to 2035.
Key Industry Insights & Findings from the Report:
Drug delivery through the skin is not a new concept; needle-and-syringe combinations have been utilized for decades. Drug delivery with extra features for reduced pain, improved medication adherence, customer interaction, user-friendly design, and convenience of administration are all part of the advanced dermatology drug delivery device concept. Among the devices available are injectors, patches, airless dispensers and bottles, dermal pumps, valves, and add-on devices for safety and better connectivity. Furthermore, the sector is being led by an increase in the use of connected devices, a growth in the number of business synergies with pharmaceutical businesses, and an increasing focus on prescription/medication adherence. Furthermore, as consumers' preferences shift toward connected devices, proprietary technologies are expected to become increasingly important differentiators for both new entrants and established organizations in the future.
Furthermore, the industry is expected to grow as protocols become more common and people become more aware of the benefits of connected devices. Stringent restrictions and concerns about data privacy, as well as a lack of awareness about modern dermatology drug delivery devices among a broad segment of the public in emerging nations, are limiting the market's growth. Connected healthcare is a game-changing notion. However, it raises worries about data privacy. Networked medical equipment, like other linked devices, are vulnerable to security breaches. The market experienced a strong rate of innovation in terms of new introductions during the period 2015-2020. Furthermore, the concept of connected medical devices is a relatively new concept that will take time to gain traction among the general public, particularly in emerging markets.
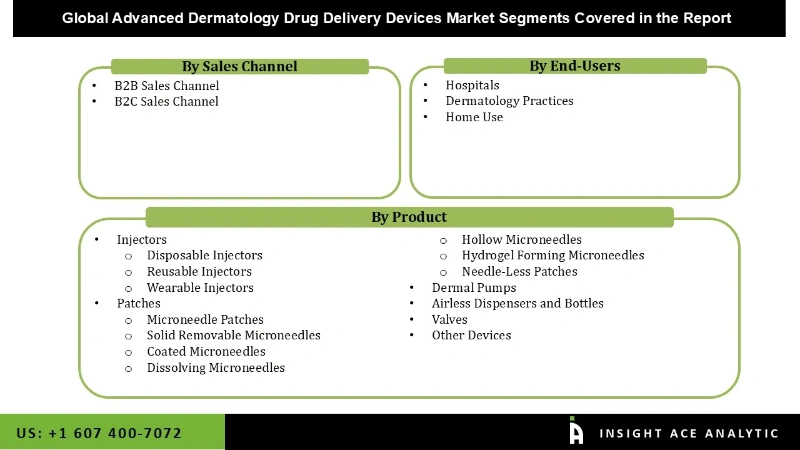
The Advanced Dermatology Drug Delivery Devices Market is segmented on the basis of Market by Products, Market by Sales Channel, and Market by End-Users. Based on Products, the market is segmented as Injectors, Disposable Injectors, Reusable Injectors, Wearable Injectors, Patches, Microneedle Patches, Solid Removable Microneedles, Coated Microneedles, Dissolving Microneedles, Hollow Microneedles, Hydrogel Forming Microneedles, Needle-Less Patches, Dermal Pumps, Airless Dispensers and Bottles, Valves, and Other Devices. By Sales Channel, the market is segmented into B2B Sales Channel, and B2C Sales Channel. By End-Users, the market is segmented into Hospitals, Dermatology Practices, and Home Use.
In 2023, injectors had the majority of the market share in the global advanced dermatological drug delivery devices market. Several innovations in product design and user involvement are boosting the injectors industry. Self-administration is also possible using injectors and autoinjectors. As a result, the convenience of use of an injector, product, or brand is essential for the performance. Furthermore, patient surveys were undertaken to identify the top factors that influence physician and patient preferences, such as comfortable grip and ease of self-injection. Some of the main companies' product specifications and unique selling points (USPs) demonstrate a great effort to ensure optimized simplicity of use while also delivering customized offers. Customization options are frequently provided for criteria including the kind of formulation, dosage, and dosage schedule. Consumer convenience is also at the forefront of innovation in the devices now under development.
Hospitals, dermatological practices, and home use are all part of the global advanced dermatology drug delivery devices market by end user. The global advanced dermatology delivery devices market's key end customers are hospitals. For immunization purposes, the use of these devices in hospitals and dermatology practices (clinics) has been steadily rising. Furthermore, the COVID-19 epidemic has aided in the acceleration of demand for connected and home-use gadgets. The segment's rise is also being fueled by developments in drug delivery technologies that promote adherence.
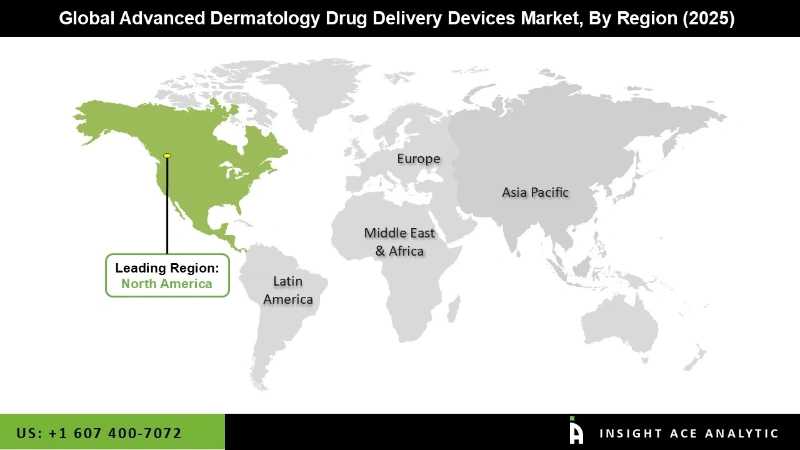
The global market for advanced dermatology drug delivery devices is expected to grow at a considerable CAGR in North America. The healthcare systems in North America are among the most technologically advanced in the world. The region boasts a considerable number of early adopters for advanced dermatology medication delivery systems, and it is the world's top market for these devices. However, Europe is the fastest-growing region during the forecast period. Europe is a crucial market for early adopters of innovative devices, and it is projected to be one of the top markets for sophisticated dermatology drug delivery systems in the future. Connected devices have grown in popularity in Europe due to the benefits they offer over traditional techniques including enhanced patient care and safety, shorter hospital stays, and faster recovery. Furthermore, the European Union is a world leader in terms of design, features, and technology. Strategic collaborations, M&As, and a strong preference for innovative medication delivery systems among end-users are projected to help the European industry.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 6.02 Billion |
| Revenue Forecast In 2035 | USD 15.86 Billion |
| Growth Rate CAGR | CAGR of 10.30% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Billion and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2025 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Product, By Sales Channel, By End-Users |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; China; Japan; South Korea; South East Asia |
| Competitive Landscape | AptarGroup, Inc., Becton, Dickinson and Company, Bespak, Credence MedSystems, Inc., E3D Elcam Drug Delivery Devices, Kindeva Drug Delivery L.P., Latch Medical, LTS Lohmann Therapie-Systeme AG, Medicsensors S.L, Midas Pharma GmbH, Nemera, Owen Mumford, Portal Instruments, Inc., Vaxxas Inc., West Pharmaceutical Services, Inc., The 3M Company, Zosano Pharma Corporation, Raphas Co., Ltd., Nanopass Tech, Corium International, Inc., Valeritas, Inc., Nitto Denko Corporation, Microdermics Inc., TheraJect, Inc., Endoderma Ltd., QuadMedicine, SNvia Co., Ltd., Small Lab, AdminMed nanoBioSciences LLC, BioSerenTach Inc, Innoture Medical, Technology Limited, Clearside Biomedical, CosMED Pharmaceutical Co.Ltd., Mindera Corp |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
By Product-
By Sales Channel-
By End-Users-
By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.