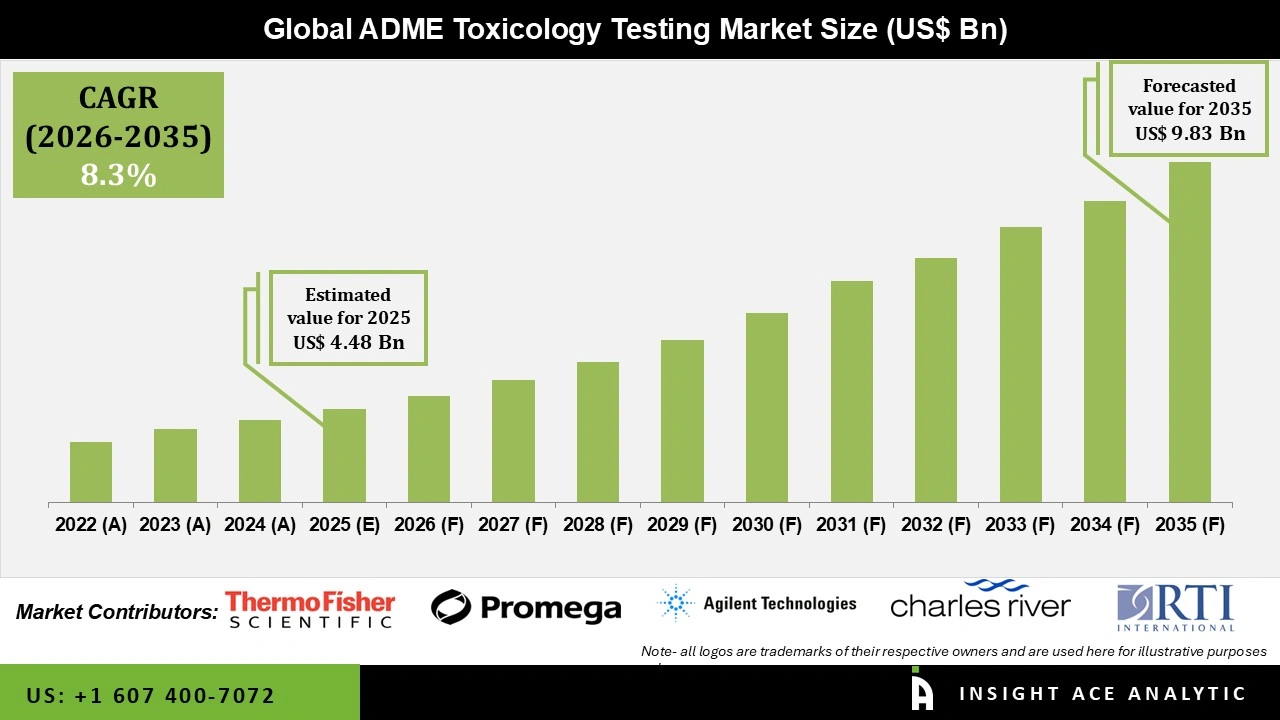
Global ADME Toxicology Testing Market Size is valued at USD 4.48 Bn in 2025 and is predicted to reach USD 9.83 Bn by the year 2035 at an 8.3% CAGR during the forecast period for 2026 to 2035.
ADME Toxicology Testing Market, Share & Trends Analysis Report, By Type of Services (Absorption, Distribution, Metabolism, Excretion), Type of Assays (Batch / Fed-Batch, Continuous), Type of Molecule (Biologics, Small Molecules), End User, Therapeutic Areas, By Region, and Segment Forecasts, 2026 to 2035
ADME, which stands for Absorption, Distribution, Metabolism, and Excretion, refers to the studies designed to investigate how a chemical, such as a drug compound, is processed by a living organism. These studies help determine how the drug is absorbed into the bloodstream, distributed throughout the body, metabolized into different substances, and excreted. Toxicology tests, which are often part of this process, provide insights into the potential adverse effects of the drug. When combined, these studies yield the acronym ADMET, which encompasses both the pharmacokinetic and toxicological evaluation necessary for assessing the safety and efficacy of new drug candidates.
ADME toxicology testing helps to identify drugs or chemicals that are likely to be toxic, have poor bioavailability (i.e., they are not well absorbed by the body), or interact with other drugs. Additionally, ADME testing aids in optimizing the dosing of drugs. By addressing these critical aspects, ADME toxicology testing ensures that new drugs are safe and effective for patients. It includes studying acute, chronic, and sub-chronic toxicity, genotoxicity, carcinogenicity, reproductive and developmental toxicity, and other specific toxicological endpoints.
The application of ADME toxicology testing in drug development and chemical safety assessment is multifaceted and crucial. It is used to determine drugs or chemicals likely to be toxic, preventing potential harm to patients and identify potential interactions with other drugs, minimizing adverse effects.
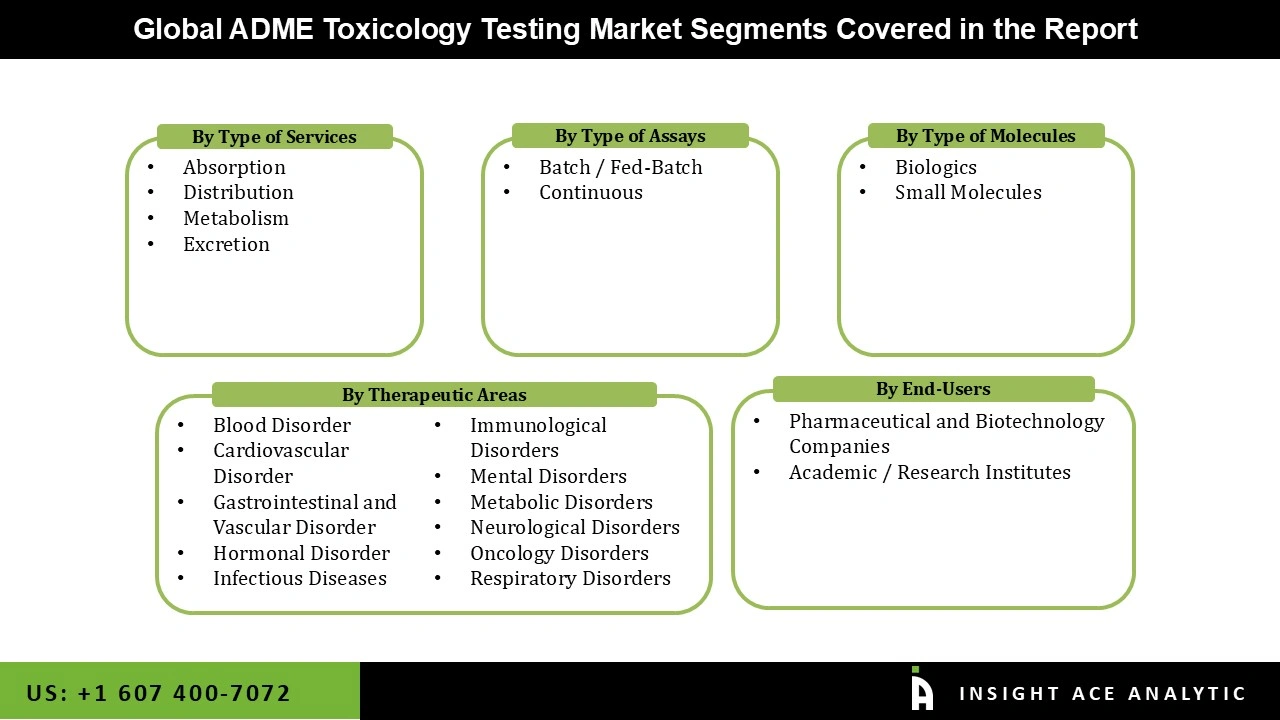
The is segmented based on the type of services, type of assays, type of molecule, end user, and therapeutic areas. By Type of Services segmented into absorption, distribution, metabolism, and excretion. Based on the Type of Assays segmented into batch / fed-batch, continuous. Therapeutic Areas, the market is categorized into blood disorder, cardiovascular disorder, gastrointestinal and vascular disorder, hormonal disorder, infectious diseases, immunological disorders, mental disorders, metabolic disorders, neurological disorders, oncology disorders, respiratory disorders. Type of Molecule segmented into biologics, small molecules. End User segmented into pharmaceutical and biotechnology companies, academic / research institutes.
Based on Therapeutic Areas, the market is categorized into blood disorder, cardiovascular disorder, gastrointestinal and vascular disorder, hormonal disorder, infectious diseases, immunological disorders, mental disorders, metabolic disorders, neurological disorders, oncology disorders, respiratory disorders. Among these, the oncology disorders segment is expected to have the highest growth rate during the forecast period. This is because cancer drug development is a highly active and rapidly evolving area of research and development. Oncology drugs often require extensive ADME testing to ensure they are effectively absorbed, distributed, metabolized, and excreted, as well as to understand their potential toxicological effects. The high prevalence and significant clinical need for effective cancer treatments drive substantial investment and focus in this segment, leading it to dominate the ADME toxicology testing market.
Based on type of Assay, the segment divided into batch / fed-batch, continuous form. Among these, the batch / fed-batch segment dominates the market during the forecast period. Batch and fed-batch processes are more commonly used in the production and testing of pharmaceuticals, including ADME toxicology studies, because they offer greater control over experimental conditions and are well-suited to the scale of most laboratory and preclinical studies. These methods allow for precise adjustments to be made to the testing environment and are easier to manage compared to continuous processes, which are more complex and typically used for large-scale manufacturing rather than initial testing and development phases.
North America, particularly the United States, has a highly developed healthcare infrastructure that supports extensive research and development activities. The region is home to many of the world's leading pharmaceutical companies, which conduct extensive drug development and testing.
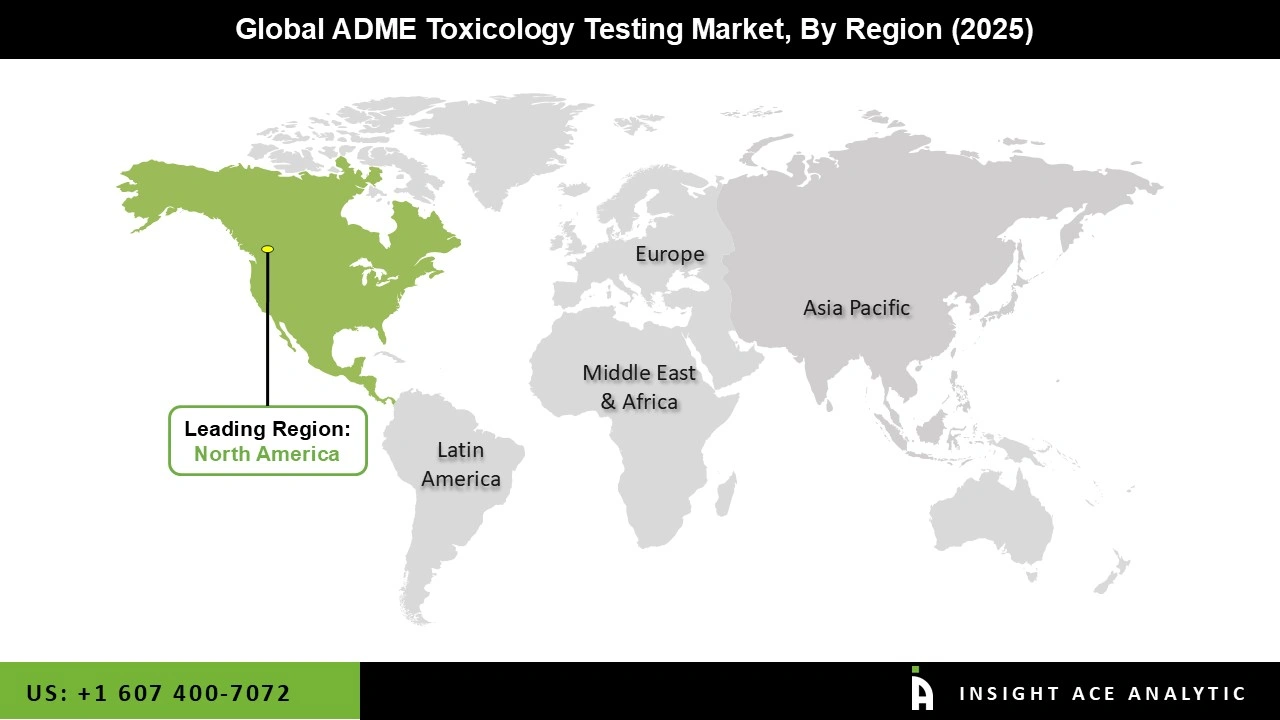
North America is at the forefront of adopting advanced technologies and methodologies in ADME toxicology testing, improving efficiency and outcomes. The availability of a highly skilled workforce in biomedical and pharmaceutical sciences enhances the capacity for high-quality ADME testing.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 4.48 Bn |
| Revenue Forecast In 2035 | USD 9.83 Bn |
| Growth Rate CAGR | CAGR of 8.3% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026 to 2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Type of Services, Type of Assays, Type of Molecule, End User, Therapeutic Areas |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; South East Asia |
| Competitive Landscape | Thermo Fisher Scientific Inc., Promega Corporation, Agilent Technologies, Inc., Charles River Laboratories, RTI International., Eurofins Scientific, Evotec, Galapagos, Tecan Group, GVK Biosciences, Pharmaron, Pharmaceutical Product Development (PPD), Sai Life Sciences, Shanghai Medicilon, Syngene International |
| Customization Scope | Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.