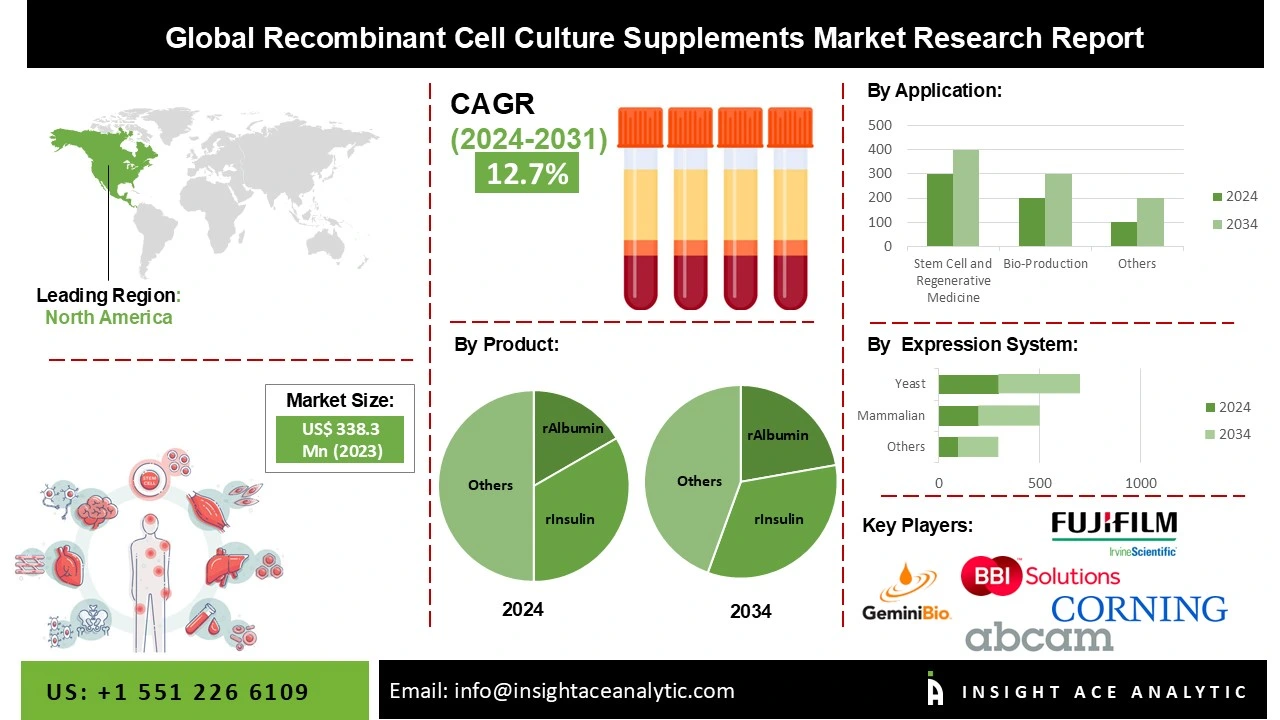
Global Recombinant Cell Culture Supplements Market Size is valued at USD 388.35 Mn in 2023 and is predicted to reach USD 1008.85 Mn by the year 2031 at a 12.75% CAGR during the forecast period for 2024 to 2031.
Recombinant Cell Culture Supplements Market Size, Share & Trends Analysis Report by Product (recombinant albumin (rAlbumin), recombinant insulin (rInsulin), recombinant epidermal growth factor (rEGF), recombinant interleukin growth factor (rILGF), recombinant transferrin (rTransferrin), recombinant trypsin (rTrypsin), recombinant Insulin-like growth factor (rIGF), recombinant stem cell factor (rSCF), recombinant aprotinin (rAprotinin), recombinant lysozyme (rLysozyme)), Application, And Expression System, Region And Segment Forecasts, 2024 to 2031
Rising demand for cell culture supplements, increased mergers and acquisitions to diversify the portfolio of recombinant cell culture supplements, and increased funding for biopharmaceutical product research and development in the life sciences sector are some factors boosting market growth. Other factors boosting market growth include rising demand for stem cell and regenerative medicine research, immunotherapy, and the need for animal-free supplements in cell culture applications. Regulatory bodies are also pushing for the use of animal-free media supplements in cell culture processes. The growth is anticipated to be inhibited by the high cost, the technical difficulties related to recombinant cell culture supplements, and the shortage of qualified personnel.
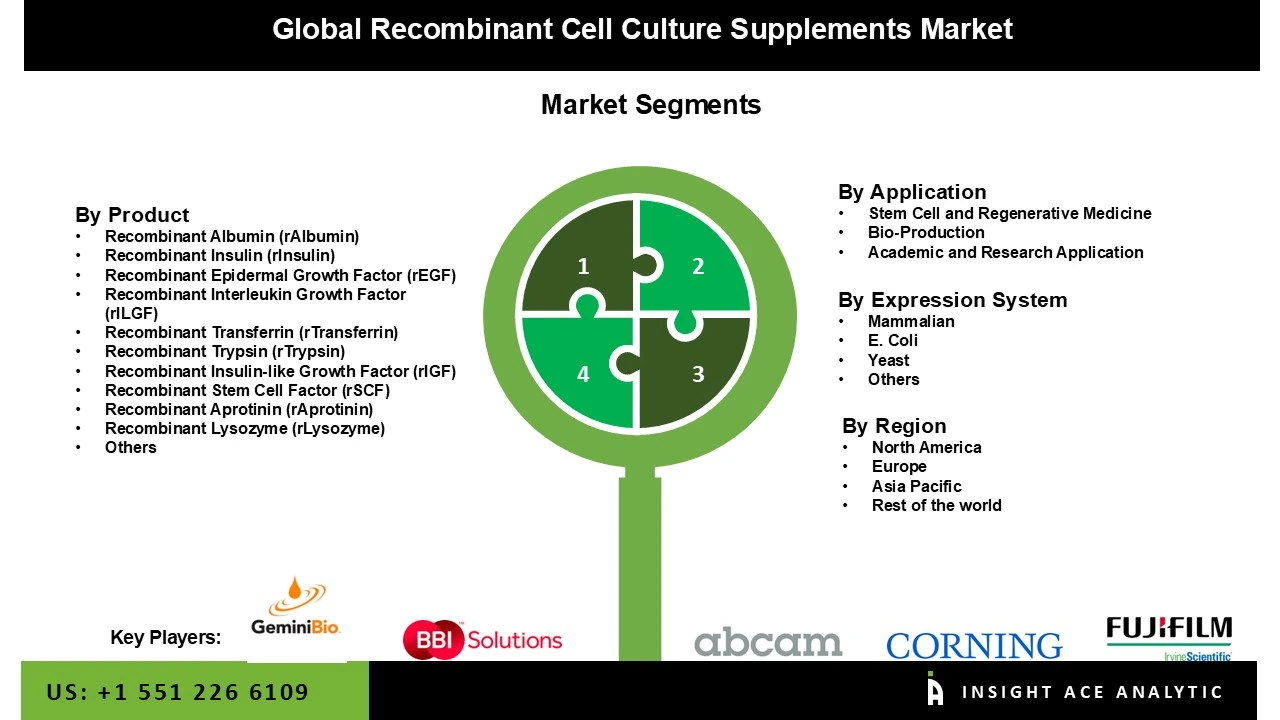
The recombinant cell culture supplements Market is segmented on the basis of product, application, and expression system. Product segment includes recombinant albumin (rAlbumin), recombinant insulin (rInsulin), recombinant epidermal growth factor (rEGF), recombinant interleukin growth factor (rILGF), recombinant transferrin (rTransferrin), recombinant trypsin (rTrypsin), recombinant Insulin-like growth factor (rIGF), recombinant stem cell factor (rSCF), recombinant aprotinin (rAprotinin), recombinant lysozyme (rLysozyme), and others. Application segment includes stem cell and regenerative medicine, Bio-Production, and academic and research application. The expression system segment includes Mammalian, E. Coli, Yeast, and others.
Stem cell and regenerative medicine held a commanding market share in 2023, leading the global market for recombinant cell culture supplements (by application). But due to improved cell viability and batch-to-batch uniformity of final products, academic and research applications are identified to be the fastest expanding segment over the forecast period.
With a significant market share in 2023, the mammalian expression system dominated the recombinant cell culture supplements market. Stable cell lines produced from mammalian cells are used by the majority of biopharmaceutical businesses in the world to produce biologics.
In 2023, North America dominated the market for supplements used in recombinant cell cultures. The expansion can be linked to the region's inclusion of essential players as well as the accessibility of money and technology for recombinant cell culture supplements.
| Report Attribute | Specifications |
| Market size value in 2023 | USD 388.35 Mn |
| Revenue forecast in 2031 | USD 1008.85 Mn |
| Growth rate CAGR | CAGR of 12.75% from 2024 to 2031 |
| Quantitative units | Representation of revenue in US$ Billion and CAGR from 2024 to 2031 |
| Historic Year | 2019 to 2023 |
| Forecast Year | 2024-2031 |
| Report coverage | The forecast of revenue, the position of the company, the competitive market statistics, growth prospects, and trends |
| Segments covered | Product, Application, And Expression System |
| Regional scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; Southeast Asia |
| Competitive Landscape | Abcam plc., Corning Incorporated, Lonza Group AG, Merck KGaA, Sartorius AG, and Thermo Fisher Scientific Inc., BBI Solutions, FUJIFILM Irvine Scientific, Inc., Gemini Bioproducts, LLC, HiMedia Laboratories, InVitria, Kingfisher Biotech, Inc., Novus Biologicals, LLC, R&D Systems, Inc., Sino Biological Inc., and STEMCELL Technologies Inc. |
| Customization scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and available payment methods | Explore pricing alternatives that are customized to your particular study requirements. |
Recombinant Cell Culture Supplements Market By Product-
Recombinant Cell Culture Supplements Market By Application-
Recombinant Cell Culture Supplements Market By Expression System-
Recombinant Cell Culture Supplements Market By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.