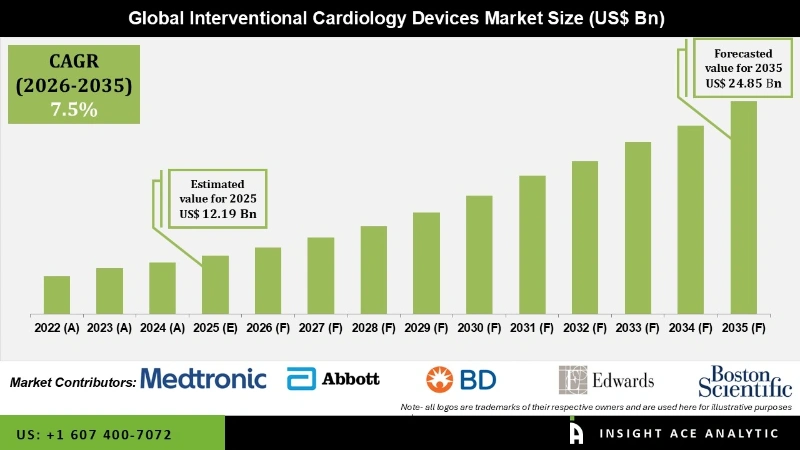
The Interventional Cardiology Devices Market Size is valued at USD 12.19 Bn in 2025 and is predicted to reach USD 24.84 Bn by the year 2035 at a 7.5% CAGR during the forecast period for 2026 to 2035.
Interventional Cardiology Devices Market Size, Share & Trends Analysis Report By Product (Angioplasty Balloons, Angioplasty Stents, Structural Heart Devics, Catheters, Plaque Modification Devices, Hemodynamic Flow Alteration Devices Other Devices), By Region, And by Segment Forecasts, 2026 to 2035.
Specialized tools are needed for interventional procedures to repair weak or damaged vessels, clogged arteries, or other impacted areas of the heart without surgery. Therefore, minimally invasive procedures are performed in hospitals or cardiac catheterization labs using interventional cardiology equipment such as coronary stents, cutting balloon catheters, and percutaneous transluminal coronary angioplasty (PTCA).
Cardiovascular diseases, like the coronary heart disease, stroke, and atrial fibrillation, are becoming more commonplace globally. The increase in biological risk factors, such as alcohol intake, bad food, cigarette use, and smoking, as well as environmental risk factors like pollution, are to blame for the increased prevalence of cardiovascular illnesses. The leading players' increasing product introductions are expected to boost the market for interventional cardiology devices.
Also, the significant occurance of cardiovascular conditions, comprising valvar defects and atrial stenosis, in developing regions like China, India, & Brazil has resulted in a strong need for interventional cardiology equipment. Major players are working together more and more to test a number of interventional devices in clinical studies. In March 2021, B. Braun SE and Infraredx collaborated to work an IDE clinical study for the drug coated PTCA balloon catheter SeQuent Please ReX.
Some Major Key Players In The Interventional Cardiology Devices Market:
Market Segmentation:
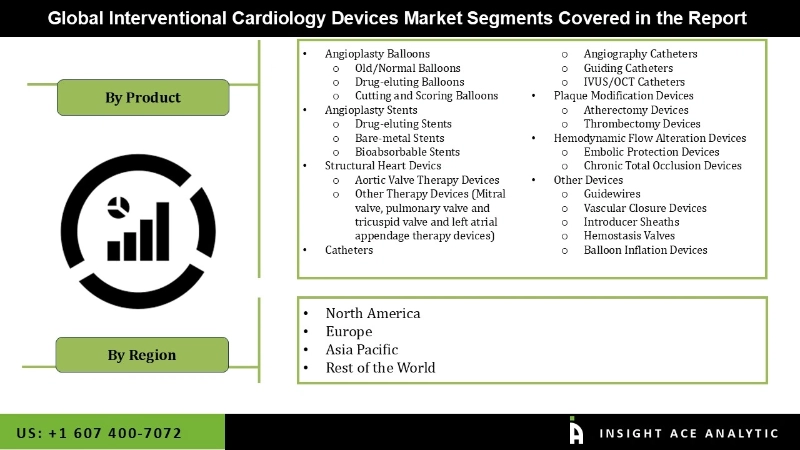
The Interventional Cardiology Devices market is divided on the basis of Product. By Product the market is again divided into Angioplasty Balloons, Angioplasty Stents, Structural Heart Devics, Catheters, Plaque Modification Devices, Hemodynamic Flow Alteration Devices Other Devices. These subsegments are againd divided into more divisions. (Angioplasty Balloons is segmented into Old/Normal Balloons, Drug-eluting Balloons, Cutting and Scoring Balloons. Whereas Angioplasty Stents segement is divided into Drug-eluting Stents, Bare-metal Stents, Bioabsorbable Stents.
The Structural Heart Devics segment comprises Aortic Valve Therapy Devices, and Other Therapy Devices like Mitral valve, pulmonary valve and tricuspid valve & left atrial appendage therapy devices)). The Catheters segment includes Angiography Catheters, Guiding Catheters, and IVUS/OCT Catheters. Whereas, Plaque Modification Devices comprises Atherectomy Devices, and Thrombectomy Devices. At the end, Hemodynamic Flow Alteration Devices is divided into Embolic Protection Devices, & Chronic Total Occlusion Devices. Lastly Other Devices are Guidewires, Vascular Closure Devices, Introducer Sheaths, Hemostasis Valves, & Balloon Inflation Devices.
Because coronary artery illnesses are so common, the sector for coronary vascular devices is expected to dominate. Because ballons and stents devices are becoming more common, structural cardiac devices are likewise predicted to have expanding growth prospects.
The embolic protection devices segment dominated the market for devices that alter the hemodynamic flow. This is due to the benefits of embolic protection devices over chronic total occlusion devices, such as the capacity to capture embolic detritus without interrupting continuous blood flow. In addition, the number of interventional procedures used to treat coronary artery disease is growing.
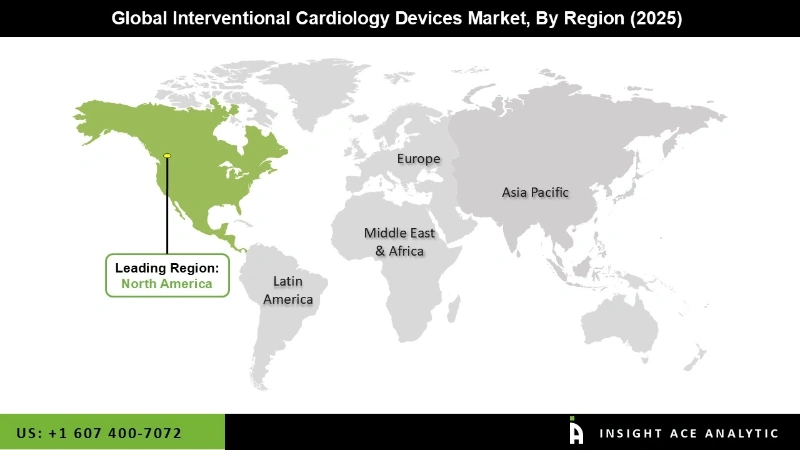
The North America Interventional Cardiology Devices market is projected to get the highest revenue share in the near future. This is because North America is showing support a high rate of medical device innovation and has a high rate of using expensive interventional cardiology devices. It is the leader in the market under study. Some big market players in the area are also making new products and technologies to fight with products that are already on the market, while others are buying and working with other businesses that are popular in the market.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 12.19 Bn |
| Revenue Forecast In 2035 | USD 24.84 Bn |
| Growth Rate CAGR | CAGR of 7.5% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Bn, Volume (No.of Units) and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | By Product |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
| Competitive Landscape | Medtronic (US), Boston Scientific Corporation (US), Abbott (US), Edward Lifescinces Corporation (US), Cardinal Health (US), iVascular (Spain), Becton, Dickinson, and Company (US), B. Braun Melsungen (Germany), Terumo Corporation (Japan), Biosensors International Group (Singapore), and BIOTRONIK SE & Co. KG (Germany) |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Interventional Cardiology Devices Market Value (US$ Mn) and Volume (No. of Units) By Product
Interventional Cardiology Devices Market Value (US$ Mn) and Volume (No. of Units) By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.