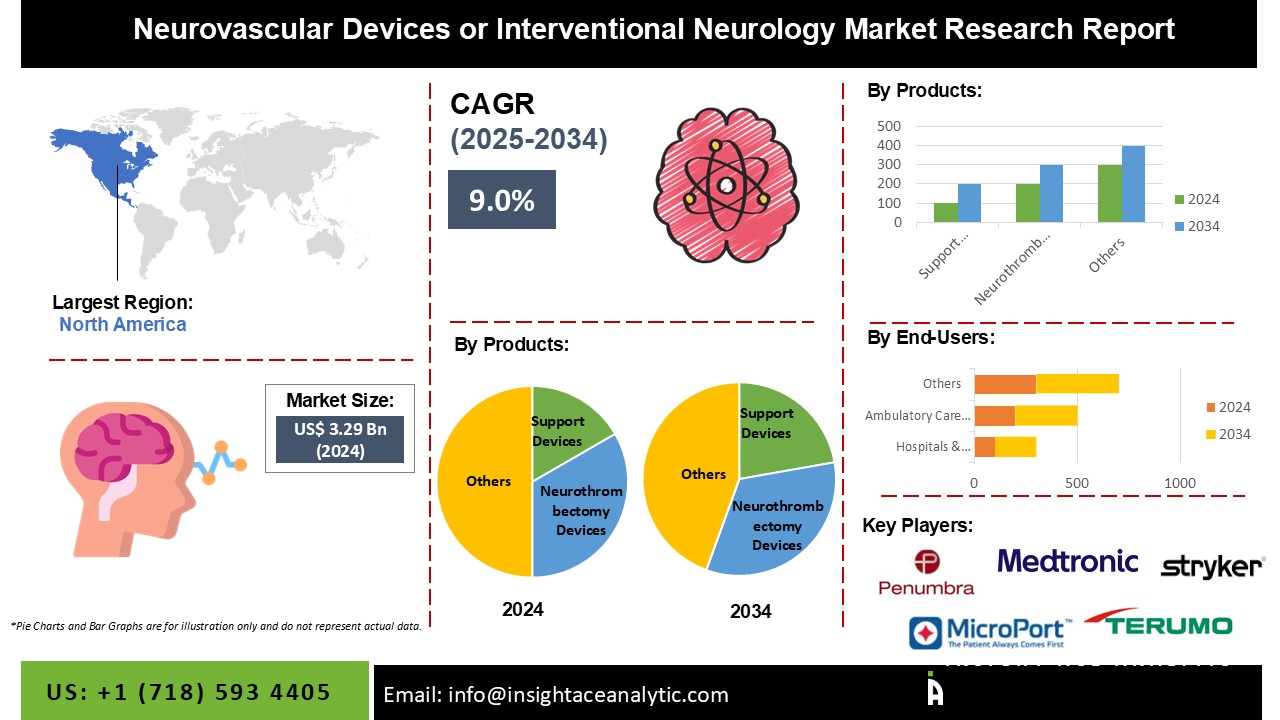
Neurovascular Devices or Interventional Neurology Market Size is valued at 3.29 Billion in 2024 and is predicted to reach 7.70 Billion by the year 2034 at a 9.0 % CAGR during the forecast period for 2025-2034.
Neurovascular conditions encompass intracranial aneurysms, ischemic stroke, subarachnoid hemorrhage, cavernous malformations, arteriovenous malformations, carotid disease, intracerebral hemorrhage, and moyamoya disease. Neurovascular devices treat a blood vessel or blood flow that impairs brain and nervous system function.
Multiple factors drive the growth of the neurovascular devices or interventional neurology market, including the increasing prevalence of neurovascular diseases, the development of new neurovascular devices, improved medical reimbursement policies, rising demand for safe and effective neurovascular devices, a well-established healthcare infrastructure, and an increase in research and development activities for neurovascular therapies. Besides this, growing patient demand for minimally invasive treatments results in the innovation and launch of neurovascular devices, which is likely to drive the growth of the neurovascular devices market. For instance, in August 2020, Stryker Corporation (US) launched the Surpass Evolve Flow Diverter and received U.S. FDA approval for the same. This diverter is the first 64-wire cobalt-chromium flow diverter in the U.S. designed to re-direct blood flow and promote aneurysm healing. However, the elevated cost of neurovascular devices and treatments may restrain the market’s growth.
The neurovascular devices or interventional neurology market is classified based on the products, end-users, and region. The products segment is categorized into aneurysm coiling & embolization devices (embolic coils, flow diversion devices, liquid embolic agents), cerebral balloon angioplasty & stenting systems (carotid artery stents, embolic protection, balloon catheters), support devices (microcatheters and guidewires), and neurothrombectomy devices (clot retrievals, suction & aspiration, snares). By end-users, the market is segmented into hospitals & surgical centres, ambulatory care centres, and research laboratories & academic institutes. The hospitals & surgical centres segment is estimated to proliferate in the coming years due to the rising surgical operations and better reimbursement policies for hospital-based interventions. Geographically, the market is studied across North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa.
North America is projected to witness lucrative growth in this market during the forecast period due to the well-developed healthcare facilities and rising prevalence of stroke & chronic diseases in this region.
| Report Attribute | Specifications |
| Market Size Value In 2024 | USD 3.29 Billion |
| Revenue Forecast In 2034 | USD 7.70 Billion |
| Growth Rate CAGR | CAGR of 9.0% from 2025 to 2034 |
| Quantitative Units | Representation of revenue in US$ Million and CAGR from 2025 to 2034 |
| Historic Year | 2021 to 2024 |
| Forecast Year | 2025-2034 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | Products, End-User |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Johnson & Johnson (US), Medtronic PLC (Ireland), Stryker Corporation (US), Terumo Corporation (Japan), Penumbra, Inc. (US), Microport Scientific Corporation (China), Kaneka Corp. (Japan), Integer Holdings Corporation (US), Balt (France), Perflow Medical (Israel), Phenox GmbH (Germany), Sensome (France), Evasc (Canada), Rapid Medical (Israel), Asahi Intecc Co. Ltd (Japan), Acandis GmbH (Germany), Medikit Co. Ltd (Japan), Imperative Care (US), Lepu Medical (China), Cerus Endovascular (US), MicroVention, Inc. (US), Mizuho Medical (Japan), Peter Lazic GmBH (Germany), Spartan Micro (US), Emboflu (Switzerland), Straub Medical AG (Switzerland), AngioDynamics (US), Microbot Medical Inc. (US) and others. Companies Mentioned |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Neurovascular Devices or Interventional Neurology Market, by Products,
Global Neurovascular Devices or Interventional Neurology Market, by End-Users,
Global Neurovascular Devices or Interventional Neurology Market, by Region,
North America Neurovascular Devices or Interventional Neurology Market, by Country,
Europe Neurovascular Devices or Interventional Neurology Market, by Country,
Asia Pacific Neurovascular Devices or Interventional Neurology Market, by Country,
Latin America Neurovascular Devices or Interventional Neurology Market, by Country,
Middle East & Africa Neurovascular Devices or Interventional Neurology Market, by Country,
Competitive Landscape
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.