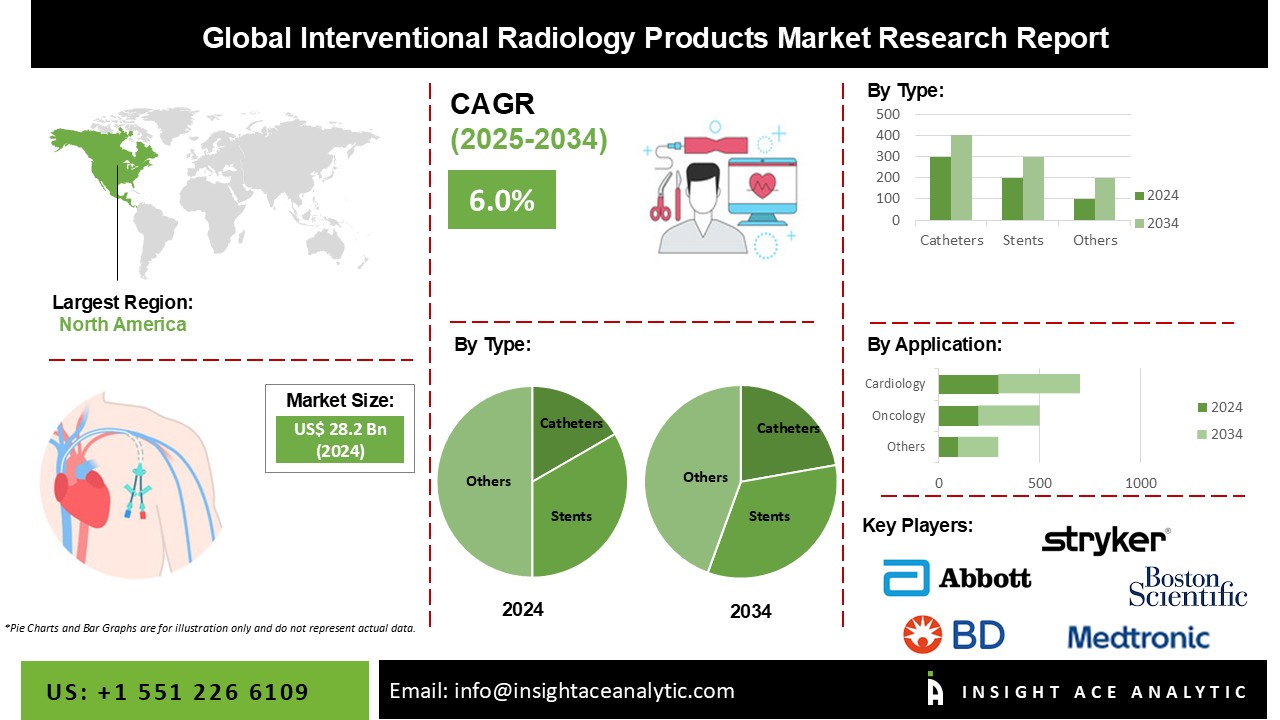
Interventional Radiology Products Market Size is valued at 28.2 Billion in 2024 and is predicted to reach 49.9 Billion by the year 2034 at a 6.0% CAGR during the forecast period for 2025-2034.
Interventional radiology is a medical speciality that focuses on using minimally invasive image-guided techniques to diagnose and treat patients. Radiological guidance systems and technologies such as x-ray, MRI, CT, fluoroscopy, and other modalities are employed in this medical specialty. The most common interventional radiological procedures conducted using these technologies are for the diagnosis of any underlying illnesses that the patient may have, which can be discovered by photography visualization. Interventional radiology is sometimes known as vascular radiology or interventional radiology (VIR). The increased prevalence of chronic diseases such as heart disease and cancer among the global population will act as the main driver, propelling the market forward. Consumer demand for minimally invasive procedures will drive up demand for interventional radiology technologies, including CT scans, MRIs, and ultrasounds, resulting in a quicker market growth rate. The growing focus on the development of innovative/digital inhalers is another major factor driving market expansion.
Furthermore, rising healthcare costs and growing demand for painless drug administration are two significant aspects that will propel the market forward. Similarly, as the number of applications grows, the market's growth rate accelerates. Rapid urbanization, changing lifestyles, and increased disposable incomes in developing and developed countries will influence the rate of growth of the interventional radiology products market. The market for interventional radiology devices would benefit from an increase in the senior population as well as good reimbursement circumstances. On the other side, the high costs of digital radiology instruments such as CT scanners, X-ray systems, and MRI scanners will stifle industry growth. High radiation exposure and tough competition among market competitors will also pose challenges to the interventional radiology products market. Furthermore, a lack of trained workers and a lack of information will break down the market's growth rate, delaying it.
The Interventional Radiology Products market is categorized on the basis of Type, Procedure and application. Based on type, the market is segmented as Catheters, Stents, Inferior Vena Cava (IVC) Filters, Hemodynamic Flow Alteration Devices, Angioplasty Balloons, Thrombectomy Systems, Embolization Devices, Biopsy Needles, Accessories, and Other Interventional Radiology Products. On the basis of Procedure market is segmented into Angioplasty, Angiography, Embolization, Thrombolysis, Biopsy & Drainage, Vertebroplasty, Nephrostomy, and Other Procedures. By applications, the market is segmented into Cardiology, Urology & Nephrology, Oncology, Gastroenterology, Neurology, Orthopedics, and Other Applications.
Cardiology, Urology & Nephrology, Oncology, Gastroenterology, Neurology, Orthopedics, and Other Applications are the market segments based on application. In 2019, the cardiology segment dominated the market. Cardiology problems are becoming increasingly common in the adult and geriatric populations. As patients grow older, the need for high-quality care to treat this illness grows as well. As a result, a number of countries are working to improve access to interventional radiology treatment.
The market is segmented based on type: Catheters, Stents, Inferior Vena Cava (IVC) Filters, Hemodynamic Flow Alteration Devices, Angioplasty Balloons, Thrombectomy Systems, Embolization Devices, Biopsy Needles, Accessories, and Other Interventional Radiology Products. Stents dominate the interventional radiology product market. The expanding incidence of cardiac illnesses and cancer, as well as the increasing number of angioplasty operations, performed globally, are responsible for the considerable share of this category.
In 2019, North America was the most important regional market for the market. The region's substantial market share is due to a high prevalence of chronic diseases, a growing elderly population, an increase in the usage of minimally invasive procedures, and the existence of prominent companies in the region. On the other hand, the interventional radiology products market in the Asia Pacific is estimated to grow at the quickest CAGR during the forecast period. The rising prevalence of chronic diseases, the growing demand for quality medical care, rising healthcare spending and government initiatives, increasing disposable income, growth in the medical device industry, and improvements in healthcare infrastructure in Southeast Asian countries are all significant factors driving the Asia Pacific market's growth.
| Report Attribute | Specifications |
| Market size value in 2024 | USD 28.2 Billion |
| Revenue forecast in 2034 | USD 49.9 Billion |
| Growth rate CAGR | CAGR of 6.0% from 2025 to 2034 |
| Quantitative units | Representation of revenue in US$ Million and CAGR from 2025 to 2034 |
| Historic Year | 2021 to 2024 |
| Forecast Year | 2025-2034 |
| Report coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments covered | By Type, By Procedure, By Application |
| Regional scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ;The UK; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Medtronic (Ireland), Boston Scientific Corporation (US), Becton, Dickinson and Company (US), Abbott (US), Cardinal Health (US), B. Braun Melsungen AG (Germany), Stryker (US), Terumo Medical Corporation (Japan), Cook Medical (US), Biosensors International Group, Ltd. (Singapore), Teleflex Incorporated (US), iVascular S.L.U. (Spain), Penumbra, Inc. (US), BIOTRONIK SE & Co. KG (Germany), ENDOCOR GmbH (Germany), Meril Life Sciences Pvt. Ltd. (India), Palex Medical (Spain), UreSil, LLC (US), Alvimedica (Turkey), Cardionovum GmbH (Germany), SMT (India), Medinol Ltd. (Israel), Comed B.V. (Netherlands), SCITECH (Brazil), Balton Sp. z o.o. (Poland), Rontis (Switzerland), and other prominent players |
| Customization scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing and available payment methods | Explore pricing alternatives that are customized to your particular study requirements. |
By Type-
By Procedure-
By Applications-
By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.