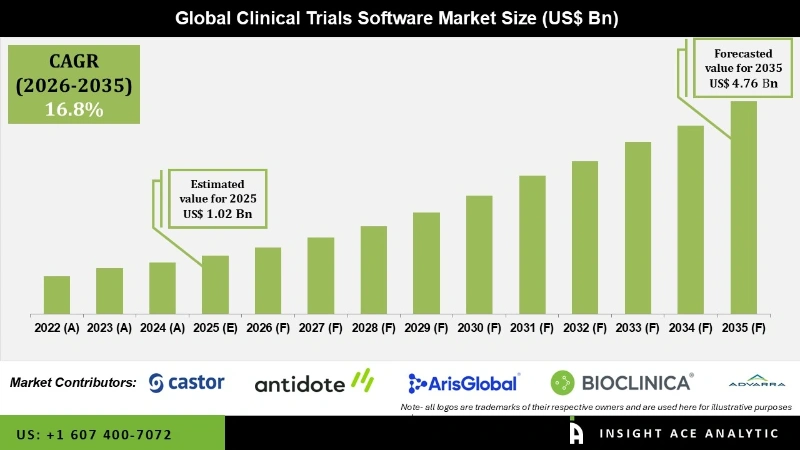
Global Clinical Trials Software Market Size is valued at USD 1.02 Billion in 2025 and is predicted to reach USD 4.76 Billion by the year 2035 at a 16.8% CAGR during the forecast period for 2026 to 2035.
Clinical Trials Software Market Size, Share & Trends Analysis Report, Type of Deployment (On-Cloud, On-Premises), Type of Delivery (Web-Based, Remote Monitoring), Type of End-User (Pharmaceuticals, Biotechnology, Cros), Features of Software (EDC, eCOA/ePRO, eConsent), Trial Design, Type of Technology. By Region, Forecasts, 2026 to 2035.
Recently pharmaceutical companies started using clinical trial management services to improve the accuracy and efficiency of clinical trials. A clinical trial is a procedure that studies new treatments and drugs to check their effects on human health.
The rising use of clinical trial management services, fast adoption of cloud-based solutions, increased healthcare expenditure, high prevalence of chronic & infectious diseases, growing government investments for clinical trial services and research are projected to augument the market growth over the forecast period. Furthermore, rising innovations of clinical trials software drive the market growth. For instance, in April 2021, Bioclinica (US) introduced the new source document management solution that combines advanced software with redaction and translation services to offer support to global research sites and trial management teams.
However, the expensive clinical trials software and limited knowledge on advanced technologies among healthcare professionals may restrict the market growth during the estimated timeframe.
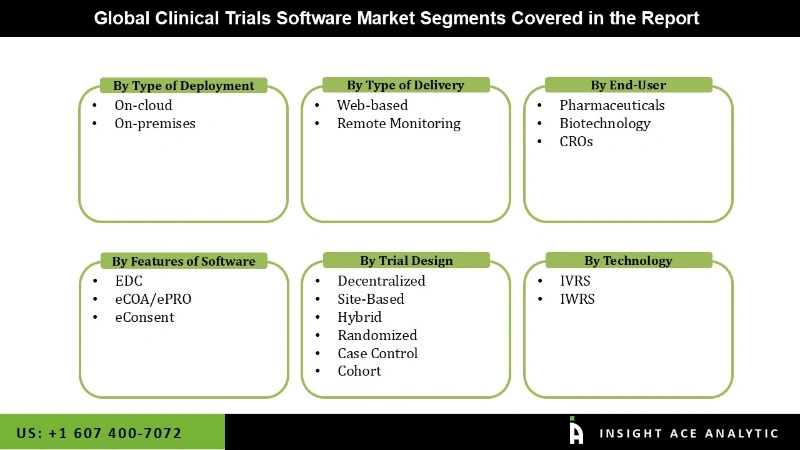
The clinical trials software market is segmented into the type of deployment, type of delivery, type of end-user, features of software, trial design, type of technology and region. The type of deployment segment comprises on-cloud and on-premises. The on-cloud segment is projected to dominate the market during the forecast period due to the surging use of cloud-based software for clinical trials. The market is classified into web-based and remote monitoring based on the type of delivery. The web-based delivery type is expected to be the fastest-growing segment of this market. It reduces R&D costs and offers cost-effective, easy accessibility to web-based clinical trial solutions.
Type of End-User, the market segmented into pharmaceuticals, biotechnology, CROs. features of software, the market segmented into EDC, eCOA/ePRO, eConsent, Trial Design the market segmented into decentralized, site-based, hybrid, randomized, case control, cohort, Type of Technology the market segmented into IVRS, and IWRS.
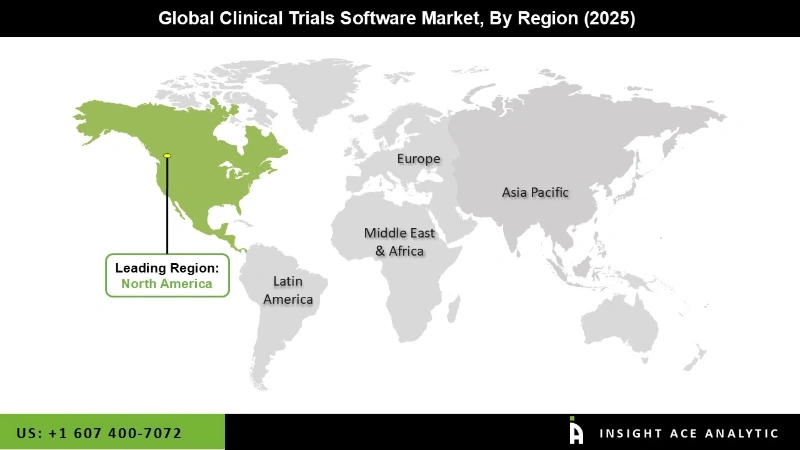
The pharmaceutical category will lead this market in the coming years due to the high demand for automated clinical trial management services. Region-wise, the market is studied across North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa.
North America dominated the clinical trials software market in 2020 and will continue its trend over the forecast period. Additionally, the Asia-Pacific market is likely to witness rapid growth in the next few years due to the growing pharmaceutical companies, increasing clinical trial studies, and high demand for web-based and cloud-based solutions for clinical trial processes.
| Report Attribute | Specifications |
| Market Size Value In 2025 | USD 1.02 Billion |
| Revenue Forecast In 2035 | USD 4.76 Billion |
| Growth Rate CAGR | CAGR of 16.8% from 2026 to 2035 |
| Quantitative Units | Representation of revenue in US$ Mn and CAGR from 2026 to 2035 |
| Historic Year | 2022 to 2024 |
| Forecast Year | 2026-2035 |
| Report Coverage | The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
| Segments Covered | Type of Deployment, Type of Delivery, Type of End-User, Features of Software, Trial Design, Type of Technology |
| Regional Scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Country Scope | U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico ; France; Italy; Spain; South Korea; South East Asia |
| Competitive Landscape | Advarra, Antidote Technologies, Inc., ArisGlobal , AssistRx, athenahealth, Inc., Axiom Real-Time Metrics, BioClinica Inc. , BSI Business Systems Integration AG, Calyx , Castor EDC, Chronicles, Clario, Clarivate, ClinCapture, Clincase, Clinical Research, CLIRINX, Cloudbyz, Dacima Software Inc., Datatrak Int. , Florence HC , IBM , Instem , IQVIA , MasterControl Inc., Medidata Solutions Inc, MedNet Solutions Inc., Novatek International, Octalsoft , Openclinica, Oracle Corporation, Parexel International Corp., RealTime Software Solutions LLC, Reify Health, Inc., Signant Health, Statsols, TrialKit , Veeva Systems Inc., WIRB-Copernicus Group, and Other prominent players. |
| Customization Scope | Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
| Pricing And Available Payment Methods | Explore pricing alternatives that are customized to your particular study requirements. |
Global Clinical Trials Software Market, by Type of Deployment,
Global Clinical Trials Software Market, by Type of Delivery,
Clinical Trials Software Market- Type of End-User
Clinical Trials Software Market- Features of Software
Clinical Trials Software Market- Trial Design
Clinical Trials Software Market Type of Technology
Global Clinical Trials Software Market, by Region,
North America Clinical Trials Software Market, by Country,
Europe Clinical Trials Software Market, by Country,
Asia Pacific Clinical Trials Software Market, by Country,
Latin America Clinical Trials Software Market, by Country,
Middle East & Africa Clinical Trials Software Market, by Country,
This study employed a multi-step, mixed-method research approach that integrates:
This approach ensures a balanced and validated understanding of both macro- and micro-level market factors influencing the market.
Secondary research for this study involved the collection, review, and analysis of publicly available and paid data sources to build the initial fact base, understand historical market behaviour, identify data gaps, and refine the hypotheses for primary research.
Secondary data for the market study was gathered from multiple credible sources, including:
These sources were used to compile historical data, market volumes/prices, industry trends, technological developments, and competitive insights.

Primary research was conducted to validate secondary data, understand real-time market dynamics, capture price points and adoption trends, and verify the assumptions used in the market modelling.
Primary interviews for this study involved:
Interviews were conducted via:
Primary insights were incorporated into demand modelling, pricing analysis, technology evaluation, and market share estimation.
All collected data were processed and normalized to ensure consistency and comparability across regions and time frames.
The data validation process included:
This ensured that the dataset used for modelling was clean, robust, and reliable.
The bottom-up approach involved aggregating segment-level data, such as:
This method was primarily used when detailed micro-level market data were available.

The top-down approach used macro-level indicators:
This approach was used for segments where granular data were limited or inconsistent.
To ensure accuracy, a triangulated hybrid model was used. This included:
This multi-angle validation yielded the final market size.
Market forecasts were developed using a combination of time-series modelling, adoption curve analysis, and driver-based forecasting tools.
Given inherent uncertainties, three scenarios were constructed:
Sensitivity testing was conducted on key variables, including pricing, demand elasticity, and regional adoption.