RNAi Therapeutics and Technology Market Size is valued at USD 1.6 Bn in 2023 and is predicted to reach USD 5.28 Bn by the year 2031 at a 16.0% CAGR during the forecast period for 2024-2031.
RNA interference (RNAi) therapeutics and technology represent a cutting-edge approach to disease treatment, utilizing the cell's natural regulatory mechanism to silence specific genes implicated in various disorders. At the core of RNAi technology lie small interfering RNA (siRNA) and microRNA (miRNA) molecules, which target and neutralize disease-causing genes with remarkable precision when introduced into the body. Through this mechanism, RNAi therapeutics offer a tailored and potent means of addressing a broad spectrum of medical conditions.
The applications of RNAi therapeutics span various diseases, ranging from genetic disorders and oncological malignancies to viral infections and neurodegenerative conditions. RNAi therapies hold promise in preventing diseases at their molecular roots by selectively silencing target genes. Additionally, RNAi technology has demonstrated efficacy in addressing cardiovascular diseases, kidney ailments, respiratory disorders, and various infectious diseases, marking a transformative shift in the landscape of medical intervention.
The increasing investments in RNAi therapy development are driving the RNAi therapeutics market forward. Driven by the acknowledgement of RNAi technology's potency in precision medicine and its expansive therapeutic potential, biotech firms and pharmaceutical companies are eagerly engaged in research and development activities, injecting substantial funds into advancing RNAi-based treatments.
The RNAi therapeutics and technology market is segmented based on type, Delivery, route of administration and end-user. By Type, the segmentation comprises Small Interfering RNA (siRNA), MicroRNA (miRNA). As per the Route of Administration, the market includes Intravenous (IV) and Subcutaneous. According to the Delivery, the market is segmented into Lipid Nanoparticles (LNPs), Conjugates, Others.
The increasing preference for siRNA (small interfering RNA) in drug development stems from its exceptional specificity in targeting disease-causing genes, potent gene silencing activity, and advancements in delivery technologies, which collectively enhance therapeutic efficacy. SiRNAs' ability to precisely silence single genes minimizes off-target effects, making them attractive for various diseases. Moreover, the rise in research collaborations and partnerships has accelerated siRNA-based therapy development, aided by successful clinical outcomes and regulatory approvals. This collaborative momentum, coupled with ongoing advancements in the field, solidifies siRNA's position as the leading segment in the RNAi market for drug development, promising novel and effective treatments for diverse medical conditions.
North America, particularly the United States, leads the RNAi therapeutics market due to its robust biotechnology sector, encompassing top-notch universities and research institutions that drive innovation in RNAi technology. Supported by favorable regulatory agencies like the FDA, the region fosters the approval and commercialization of RNAi-based therapies. High healthcare expenditure levels and well-established healthcare systems further bolster the adoption of innovative treatments. Additionally, key players and collaborative efforts among biopharmaceutical companies and academic institutions actively propel the development and commercialization of RNAi-based treatments, solidifying North America's dominance in the global RNAi therapeutics market.
|
Report Attribute |
Specifications |
|
Market Size Value In 2023 |
USD 1.6 Bn |
|
Revenue Forecast In 2031 |
USD 5.28 Bn |
|
Growth Rate CAGR |
CAGR of 16.0 % from 2024 to 2031 |
|
Quantitative Units |
Representation of revenue in US$ Bn and CAGR from 2024 to 2031 |
|
Historic Year |
2019 to 2023 |
|
Forecast Year |
2024-2031 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Type, By Delivery, By Route of Administration, |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; France; Italy; Spain; South Korea; Southeast Asia |
|
Competitive Landscape |
Alnylam, Novartis, Novo Nordisk, Regulus therapeutics, miRagen therapeutics, Roche, Santaris, Sanofi Genzyme, AstraZeneca, Viridian, Mirna Therapeutics, Arbutus Biopharma, OliX Pharmaceuticals, Inc, and Others |
|
Customization Scope |
Free customization report with the procurement of the report, Modifications to the regional and segment scope. Geographic competitive landscape. |
|
Pricing and Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global RNAi Therapeutics and Technology Market Snapshot
Chapter 4. Global RNAi Therapeutics and Technology Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. Competitive Landscape & Market Share Analysis
4.8. Qualitative Information of Technology Platform
4.8.1. Conjugated Delivery Technologies
4.8.1.1. Targeted RNAi Molecule (TRiM) Platform
4.8.1.2. GalXC Conjugated RNAi Technology Platform
4.8.1.3. The Vigil Platform
4.8.1.4. mRNAi GOLD Platform
4.8.2. Centyrin technology
4.8.2.1. Fatty Acid Ligand Conjugated OligoNucleotide (FALCON) platform
4.8.2.2. TERA platform
4.8.2.3. CASi platform
4.8.2.4. GalNAc-RNAi platform
4.9. Impact of Covid-19 Analysis
Chapter 5. Market Segmentation 1: by Type Estimates & Trend Analysis
5.1. by Type & Market Share, 2019 & 2031
5.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Type:
5.2.1. Small interfering RNA (siRNA)
5.2.2. MicroRNA (miRNA)
Chapter 6. Market Segmentation 2: by Route of Administration Estimates & Trend Analysis
6.1. by Route of Administration & Market Share, 2019 & 2031
6.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Route of Administration:
6.2.1. Intravenous (IV)
6.2.2. Subcutaneous
Chapter 7. Market Segmentation 3: by Delivery Estimates & Trend Analysis
7.1. by Delivery & Market Share, 2019 & 2031
7.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Delivery:
7.2.1. Lipid Nanoparticles (LNPs)
7.2.2. Conjugates
7.2.3. Others
Chapter 8. RNAi Therapeutics and Technology Market Segmentation 4: Regional Estimates & Trend Analysis
8.1. North America
8.1.1. North America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Type, 2023-2031
8.1.2. North America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Delivery, 2023-2031
8.1.3. North America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Route of Administration, 2023-2031
8.1.4. North America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
8.2. Europe
8.2.1. Europe RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Type, 2023-2031
8.2.2. Europe RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Delivery, 2023-2031
8.2.3. Europe RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Route of Administration, 2023-2031
8.2.4. Europe RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
8.3. Asia Pacific
8.3.1. Asia Pacific RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Type, 2023-2031
8.3.2. Asia Pacific RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Delivery, 2023-2031
8.3.3. Asia-Pacific RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Route of Administration, 2023-2031
8.3.4. Asia Pacific RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
8.4. Latin America
8.4.1. Latin America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Type, 2023-2031
8.4.2. Latin America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Delivery, 2023-2031
8.4.3. Latin America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Route of Administration, 2023-2031
8.4.4. Latin America RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
8.5. Middle East & Africa
8.5.1. Middle East & Africa RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Type, 2023-2031
8.5.2. Middle East & Africa RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Delivery, 2023-2031
8.5.3. Middle East & Africa RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by Route of Administration, 2023-2031
8.5.4. Middle East & Africa RNAi Therapeutics and Technology Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
Chapter 9. Competitive Landscape
9.1. Major Mergers and Acquisitions/Strategic Alliances
9.2. Company Profiles
9.2.1. Alnylam
9.2.2. Novartis
9.2.3. Novo Nordisk
9.2.4. Regulus therapeutics
9.2.5. miRagen therapeutics
9.2.6. Roche
9.2.7. Santaris
9.2.8. Sanofi Genzyme
9.2.9. AstraZeneca
9.2.10. Viridian
9.2.11. Mirna Therapeutics
9.2.12. Arbutus Biopharma
9.2.13. OliX Pharmaceuticals, Inc
9.2.14. Others
RNAi Therapeutics and Technology Market by Type -
RNAi Therapeutics and Technology Market by Route of Administration -
RNAi Therapeutics and Technology Market by Delivery-
RNAi Therapeutics and Technology Market by Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
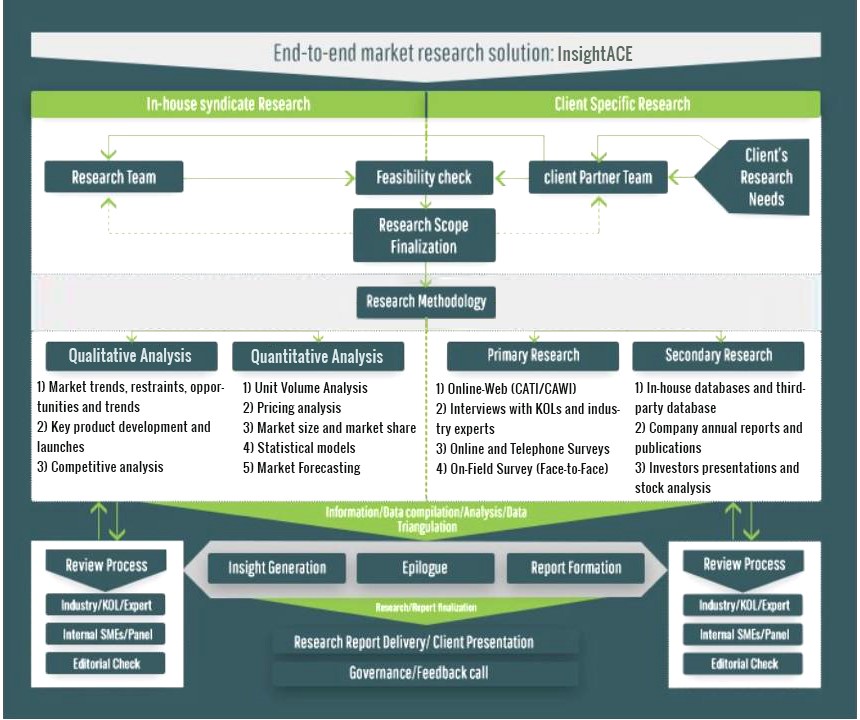
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.