Global RNA Therapy Clinical Trials Market Size is valued at USD 2.5 Bn in 2024 and is predicted to reach USD 3.6 Bn by the year 2034 at a 3.8% CAGR during the forecast period for 2025-2034.
The process of testing and evaluating the safety and effectiveness of therapeutic interventions based on RNA molecules is referred to as RNA therapy clinical trials. RNA therapy is a large group of treatments that use various forms of RNA molecules to target and modulate gene expression, such as messenger RNA (mRNA), small interfering RNA (siRNA), and antisense oligonucleotides. Various factors influence the market dynamics for RNA treatment clinical trials. As interest in and investment in RNA therapy has grown, so has competition among businesses developing RNA-based medications.
Established pharmaceutical businesses, as well as startups and biotech companies, entered the industry, spurring innovation and clinical trial activity. However, due to the pandemic, many active RNA treatment clinical trials were either halted or delayed. Clinical study activities were halted due to lockdowns, travel restrictions, and worries about the safety of trial participants and staff.
The RNA Therapy Clinical Trials Market is segmented on the basis of modality, clinical trials, and therapeutic areas. Modality segment includes RNA interference, Antisense therapy, Messenger RNA, and Oligonucleotide, non-antisense, non-RNAi. The clinical trials segment includes Phase I, Phase II, Phase III, and Phase IV. By therapeutic areas, the market is segmented into Rare Diseases, Anti-infective, anticancer, Neurological, Alimentary/Metabolic, Musculoskeletal, Cardiovascular, Respiratory, Sensory, and Others.
The Messenger RNA category is expected to hold a major share of the global RNA Therapy Clinical Trials Market in 2022. The COVID-19 pandemic pushed the development and deployment of mRNA-based vaccines, particularly Pfizer-BioNTech and Moderna's COVID-19 vaccines. Because of the effectiveness of these mRNA vaccines, mRNA technology has received attention and demonstrated potential in the fight against infectious diseases. Furthermore, mRNA therapies have various potential applications in a variety of disease conditions. They can be used to encode specific proteins that produce therapeutic antibodies, trigger an immune response, or restore protein function.
The Phase II segment is projected to grow at a rapid rate in the global RNA Therapy Clinical Trials Market. Increasing R&D investment and increasing non-industry-sponsored and industry-sponsored phase II clinical studies are important contributors to the segment's growth. Phase 2 studies enable the optimal dose and dosing schedule for RNA treatments to be determined.
The North American RNA Therapy Clinical Trials Market is expected to report the highest market share in terms of revenue in the near future. This is due to the early acceptance of RNA therapeutics, a strong research infrastructure, a favorable regulatory environment, and a large biotechnology and pharmaceutical industry. Furthermore, numerous RNA therapies have previously acquired FDA approval, and many are currently through various phases of clinical research to demonstrate their efficacy for a variety of illnesses.
Over the projection period, Asia Pacific is expected to increase significantly. Academic institutions, research organizations, and biotech firms are actively involved in RNA-based research in countries such as China, Japan, South Korea, and Singapore, resulting in an expanding pipeline of potential RNA therapeutics and an increase in the number of clinical studies in the field.
|
Report Attribute |
Specifications |
|
Market Size Value In 2024 |
USD 2.5 Bn |
|
Revenue Forecast In 2034 |
USD 3.6 Bn |
|
Growth Rate CAGR |
CAGR of 3.8% from 2025 to 2034 |
|
Quantitative Units |
Representation of revenue in US$ Bn and CAGR from 2025 to 2034 |
|
Historic Year |
2021 to 2024 |
|
Forecast Year |
2025-2034 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Modality, Clinical Trials, And Therapeutic Areas |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; South East Asia |
|
Competitive Landscape |
IQVIA; ICON Plc; Laboratory Corporation of America Holdings; Charles River Laboratories International, Inc.; PAREXEL International Corp.; Syneos Health; Medpace Holdings, Inc.; PPD Inc.; Novotech; Veristat, LLC. |
|
Customization Scope |
Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing And Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global RNA Therapy Clinical Trials Market Snapshot
Chapter 4. Global RNA Therapy Clinical Trials Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. COVID-19 Impact Analysis
Chapter 5. Market Segmentation 1: By Modality Estimates & Trend Analysis
5.1. By Modality & Market Share, 2024 -2034
5.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2021 to 2034 for the following By Modality:
5.2.1. RNA interference
5.2.2. Antisense therapy
5.2.3. Messenger RNA
5.2.4. Oligonucleotide, non-antisense, non-RNAi
Chapter 6. Market Segmentation 2: By Clinical Trials Phase Estimates & Trend Analysis
6.1. By Modality & Market Share, 2024 -2034
6.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2021 to 2034 for the following By Clinical Trials Phase:
6.2.1. Phase I
6.2.2. Phase II
6.2.3. Phase III
6.2.4. Phase IV
Chapter 7. Market Segmentation 3: By Therapeutic Areas Estimates & Trend Analysis
7.1. By Modality & Market Share, 2024 -2034
7.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2021 to 2034 for the following By Therapeutic Areas:
7.2.1. Rare Diseases
7.2.2. Anti-infective
7.2.3. Anticancer
7.2.4. Neurological
7.2.5. Alimentary/Metabolic
7.2.6. Musculoskeletal
7.2.7. Cardiovascular Respiratory
7.2.8. Sensory
7.2.9. Others
Chapter 8. RNA Therapy Clinical Trials Market Segmentation 5: Regional Estimates & Trend Analysis
8.1. North America
8.1.1. North America RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Modality, 2021-2034
8.1.2. North America RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Clinical Trials Phase, 2021-2034
8.1.3. North America RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Therapeutic Areas, 2021-2034
8.1.4. North America RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts by country, 2021-2034
8.1.4.1. U.S.
8.1.4.2. Canada
8.2. Europe
8.2.1. Europe RNA Therapy Clinical Trials Market revenue (US$ Million) By Modality, 2021-2034
8.2.2. Europe RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Clinical Trials Phase, 2021-2034
8.2.3. Europe RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Therapeutic Areas 2021-2034
8.2.4. Europe RNA Therapy Clinical Trials Market revenue (US$ Million) by country, 2021-2034
8.2.4.1. Germany
8.2.4.2. Poland
8.2.4.3. France
8.2.4.4. Italy
8.2.4.5. Spain
8.2.4.6. UK
8.2.4.7. Rest of Europe
8.3. Asia Pacific
8.3.1. Asia Pacific RNA Therapy Clinical Trials Market revenue (US$ Million) by Modality, 2021-2034
8.3.2. Asia Pacific RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Clinical Trials Phase, 2021-2034
8.3.3. Asia Pacific RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Therapeutic Areas, 2021-2034
8.3.4. Asia Pacific RNA Therapy Clinical Trials Market revenue (US$ Million) by country, 2021-2034
8.3.4.1. China
8.3.4.2. India
8.3.4.3. Japan
8.3.4.4. Australia
8.3.4.5. Rest of Asia Pacific
8.4. Latin America
8.4.1. Latin America RNA Therapy Clinical Trials Market revenue (US$ Million) by Modality, 2021-2034
8.4.2. Latin America RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts Clinical Trials Phase 2021-2034
8.4.3. Latin America RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Therapeutic Areas, 2021-2034
8.4.4. Latin America RNA Therapy Clinical Trials Market revenue (US$ Million) by country, (US$ Million) 2021-2034
8.4.4.1. Brazil
8.4.4.2. Rest of Latin America
8.5. Middle East & Africa
8.5.1. Middle East & Africa RNA Therapy Clinical Trials Market revenue (US$ Million) By Modality, (US$ Million)
8.5.2. Middle East & Africa RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Clinical Trials Phase, 2021-2034
8.5.3. Middle East & Africa RNA Therapy Clinical Trials Market revenue (US$ Million) estimates and forecasts By Therapeutic Areas, 2021-2034
8.5.4. Middle East & Africa RNA Therapy Clinical Trials Market revenue (US$ Million) by country, (US$ Million) 2021-2034South Africa
8.5.4.1. GCC Countries
8.5.4.2. Rest of MEA
Chapter 9. Competitive Landscape
9.1. Major Mergers and Acquisitions/Strategic Alliances
9.2. Company Profiles
9.2.1. IQVIA
9.2.2. ICON Plc
9.2.3. Laboratory Corporation of America Holdings
9.2.4. Charles River Laboratories International, Inc.
9.2.5. PAREXEL International Corp.
9.2.6. Syneos Health
9.2.7. Medpace Holdings, Inc.
9.2.8. PPD Inc.
9.2.9. Novotech
9.2.10. Veristat, LLC.
RNA Therapy Clinical Trials Market By Modality-
RNA Therapy Clinical Trials Market By Clinical Trials Phase-
RNA Therapy Clinical Trials Market By Therapeutic Areas-
RNA Therapy Clinical Trials Market By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
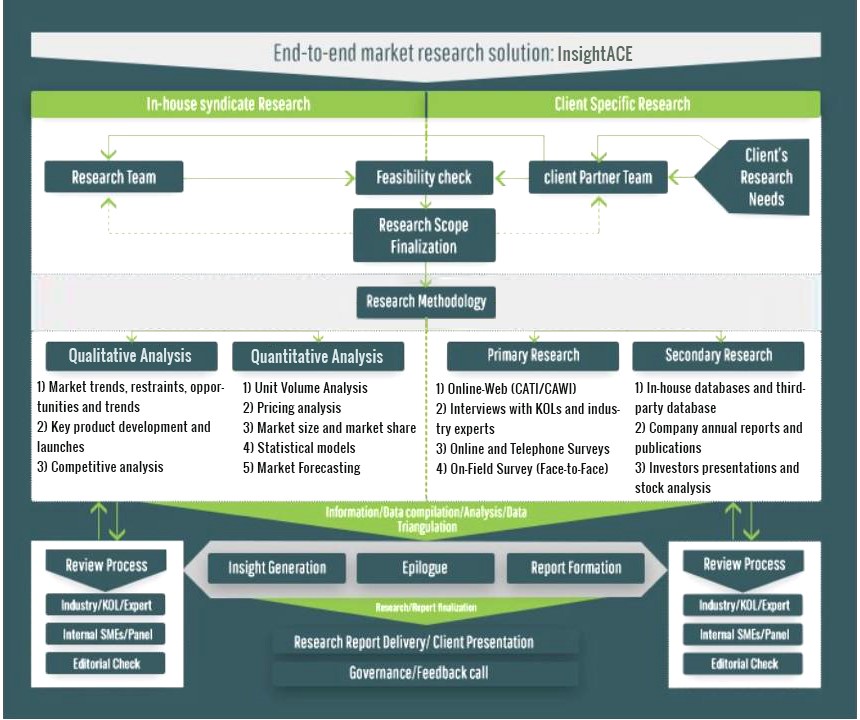
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.