Global Pharmacovigilance Market Size is valued at USD 7.8 Bn in 2024 and is predicted to reach USD 15.3 Bn by the year 2034 at a 7.1% CAGR during the forecast period for 2025-2034.
Key Industry Insights & Findings from the Report:
The pharmacovigilance market refers to the global industry of monitoring and assessing the safety and efficacy of drugs and medical products after being approved. The market includes various services and solutions, such as adverse event reporting, signal detection, risk management, regulatory compliance, and post-market surveillance.
In recent years, the pharmacovigilance market has experienced substantial growth due to the rising prevalence of chronic diseases, rising demand for personalized medicines, and growing regulatory requirements for drug safety. Advancements also drive the market in healthcare technologies, such as artificial intelligence, big data analytics, and blockchain, which have enhanced the efficiency and accuracy of pharmacovigilance processes.
Despite the increasing focus on drug safety, there is still a lack of awareness about pharmacovigilance among healthcare professionals and the general public. This could result in underreporting adverse drug reactions and delays in detecting safety signals.
The pharmacovigilance market is segmented based on the service provider, product life cycle, type, process flow, therapeutic area, and end-use. Based on the service provider, the market is segmented as in-house and contract outsourcing. Based on the product life cycle, the market is segmented into pre-clinical, phase I, phase II, phase III and phase IV. Based on type, the market is segmented into spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring and EHR mining.
Based on process flow, the market is segmented into case data management, case logging, case data analysis, medical reviewing & reporting signal detection, adverse event logging, adverse event analysis, adverse event review & reporting, risk management system, risk evaluation system and risk mitigation system. Based on therapeutic areas, the market is segmented into oncology, neurology, cardiology, and respiratory systems. Based on end-use, the market is segmented into pharmaceuticals, biotechnology companies, medical device manufacturers and others.
Contract outsourcing is one of the dominant segments in the pharmacovigilance market, and it is anticipated to continue dominating the demand in the coming years. Contract outsourcing involves outsourcing pharmacovigilance services to third-party service providers, such as contract research organizations (CROs) and business process outsourcing (BPO) companies. Factors such as cost-effectiveness, improved efficiency, and access to specialized expertise drive the contract outsourcing segment. Outsourcing pharmacovigilance services enables pharmaceutical companies to focus on their core competencies while leveraging the expertise of specialized service providers to manage the complexities of drug safety.
Moreover, outsourcing pharmacovigilance services helps companies to reduce costs associated with hiring and training personnel, managing infrastructure, and maintaining compliance with regulatory requirements. This has led to an increasing trend of outsourcing pharmacovigilance services to third-party service providers, especially among small and mid-sized pharmaceutical companies.
Spontaneous reporting is an essential component of pharmacovigilance, and it is expected to continue dominating the pharmacovigilance market in the coming years. The spontaneous reporting segment is driven by various factors, such as the widespread use of drugs and medical products, increasing awareness about drug safety, and the need for early detection and management of ADRs. Spontaneous reporting plays a crucial role in identifying and assessing the safety of drugs and medical products after being approved.
The Asia-Pacific pharmacovigilance market is expected to register the highest market share in revenue shortly. The Asia-Pacific region is anticipated to dominate the pharmacovigilance market in the coming years. The region is expected to exhibit the highest growth rate due to various factors, such as the increasing demand for pharmaceuticals, rising prevalence of chronic diseases, growing regulatory requirements, and rising awareness about drug safety and adverse effects.
Countries like China and India have been experiencing significant growth in their pharmaceutical industries, leading to increased demand for pharmacovigilance services. Moreover, the growing emphasis on patient safety and the need for regulatory compliance has further boosted the demand for pharmacovigilance solutions in the region. Besides, North America is currently the largest market for pharmacovigilance and is anticipated to continue dominating the market in the coming years. Various factors, including many pharmaceutical companies, advanced healthcare infrastructure, and increasing demand for personalized medicine, drive the North American pharmacovigilance market. The region has stringent regulatory requirements for drug safety, leading to the establishment of a well-developed pharmacovigilance infrastructure.
|
Report Attribute |
Specifications |
|
Market size value in 2024 |
USD 7.8 Bn |
|
Revenue forecast in 2034 |
USD 15.3 Bn |
|
Growth rate CAGR |
CAGR of 7.1% from 2025 to 2034 |
|
Quantitative units |
Representation of revenue in US$ Bn, and CAGR from 2025 to 2034 |
|
Historic Year |
2021 to 2024 |
|
Forecast Year |
2025-2034 |
|
Report coverage |
The forecast of revenue, the position of the company, the competitive market statistics, growth prospects, and trends |
|
Segments covered |
Service Provider, Product Life Cycle, Type, Process Flow, Therapeutic Area, And End-Use |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; Southeast Asia |
|
Competitive Landscape |
Accenture; LinicalAccelovance; Cognizant; Laboratory Corporation of America Holdings; IBM Corporation; ArisGlobal; ICON plc.; Capgemini; ITClinical; FMD K&L; IQVIA; TAKE Solutions Ltd.; PAREXEL International Corporation; BioClinica Inc.; Wipro Ltd.; and United BioSource Corporation |
|
Customization scope |
Free customization report with the procurement of the report, Modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing and available payment methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global Pharmacovigilance Market Snapshot
Chapter 4. Global Pharmacovigilance Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. Competitive Landscape & Market Share Analysis
4.8. Impact of Covid-19 Analysis
Chapter 5. Market Segmentation 1: by Product Life Cycle Estimates & Trend Analysis
5.1. by Product Life Cycle & Market Share, 2024 & 2034
5.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2021 to 2034 for the following by Product Life Cycle:
5.2.1. Pre-clinical
5.2.2. Phase I
5.2.3. Phase II
5.2.4. Phase III
5.2.5. Phase IV
Chapter 6. Market Segmentation 2: by Service Provider Estimates & Trend Analysis
6.1. by Service Provider & Market Share, 2024 & 2034
6.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2021 to 2034 for the following by Service Provider:
6.2.1. In-house
6.2.2. Contract Outsourcing
Chapter 7. Market Segmentation 3: by Type Estimates & Trend Analysis
7.1. by Type & Market Share, 2024 & 2034
7.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2021 to 2034 for the following by Type:
7.2.1. Spontaneous Reporting
7.2.2. Intensified ADR Reporting
7.2.3. Targeted Spontaneous Reporting
7.2.4. Cohort Event Monitoring
7.2.5. EHR Mining
Chapter 8. Market Segmentation 4: by Process Flow Estimates & Trend Analysis
8.1. by Process Flow & Market Share, 2024 & 2034
8.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2021 to 2034 for the following by Process Flow:
8.2.1. Case Data Management
8.2.1.1. Case Logging
8.2.1.2. Case Data Analysis
8.2.1.3. Medical Reviewing & Reporting
8.2.2. Signal Detection
8.2.2.1. Adverse Event Logging
8.2.2.2. Adverse Event Analysis
8.2.2.3. Adverse Event Review & Reporting
8.2.3. Risk Management System
8.2.3.1. Risk Evaluation System
8.2.3.2. Risk Mitigation System
Chapter 9. Market Segmentation 5: by Therapeutic Area Estimates & Trend Analysis
9.1. by Therapeutic Area & Market Share, 2024 & 2034
9.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2021 to 2034 for the following by Therapeutic Area:
9.2.1. Oncology
9.2.2. Neurology
9.2.3. Cardiology
9.2.4. Respiratory Systems
9.2.5. Others
Chapter 10. Market Segmentation 6: by End-use Estimates & Trend Analysis
10.1. by End-use & Market Share, 2024 & 2034
10.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2021 to 2034 for the following by End-use:
10.2.1. Pharmaceuticals
10.2.2. Biotechnology Companies
10.2.3. Medical Device Manufacturers
10.2.4. Others
Chapter 11. Pharmacovigilance Market Segmentation 7: Regional Estimates & Trend Analysis
11.1. North America
11.1.1. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Product Life Cycle, 2021-2034
11.1.2. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Service Provider, 2021-2034
11.1.3. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Type, 2021-2034
11.1.4. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Process Flow, 2021-2034
11.1.5. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Therapeutic Area, 2021-2034
11.1.6. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by End-use, 2021-2034
11.1.7. North America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by country, 2021-2034
11.2. Europe
11.2.1. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Product Life Cycle, 2021-2034
11.2.2. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Service Provider, 2021-2034
11.2.3. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Type, 2021-2034
11.2.4. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Process Flow, 2021-2034
11.2.5. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Therapeutic Area, 2021-2034
11.2.6. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by End-use, 2021-2034
11.2.7. Europe Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by country, 2021-2034
11.3. Asia Pacific
11.3.1. Asia Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Product Life Cycle, 2021-2034
11.3.2. Asia Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Service Provider, 2021-2034
11.3.3. Asia-Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Type, 2021-2034
11.3.4. Asia-Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Process Flow, 2021-2034
11.3.5. Asia Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Therapeutic Area, 2021-2034
11.3.6. Asia Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by End-use, 2021-2034
11.3.7. Asia Pacific Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by country, 2021-2034
11.4. Latin America
11.4.1. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Product Life Cycle, 2021-2034
11.4.2. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Service Provider, 2021-2034
11.4.3. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Type, 2021-2034
11.4.4. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Process Flow, 2021-2034
11.4.5. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Therapeutic Area, 2021-2034
11.4.6. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by End-use, 2021-2034
11.4.7. Latin America Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by country, 2021-2034
11.5. Middle East & Africa
11.5.1. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Product Life Cycle, 2021-2034
11.5.2. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Service Provider, 2021-2034
11.5.3. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Type, 2021-2034
11.5.4. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Process Flow, 2021-2034
11.5.5. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by Therapeutic Area, 2021-2034
11.5.6. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by End-use, 2021-2034
11.5.7. Middle East & Africa Pharmacovigilance Market Revenue (US$ Million) Estimates and Forecasts by country, 2021-2034
Chapter 12. Competitive Landscape
12.1. Major Mergers and Acquisitions/Strategic Alliances
12.2. Company Profiles
12.2.1. Meta Service Providers, Inc.
12.2.2. Tencent Holdings Ltd.
12.2.3. ByteDance Ltd.
12.2.4. NetEase, Inc.
12.2.5. Nvidia Corporation
12.2.6. Epic Games, Inc.
12.2.7. Roblox Corporation
12.2.8. Unity Technologies, Inc.
12.2.9. Lilith Games
12.2.10. Nextech AR Solutions Corp.
12.2.11. The Sandbox
12.2.12. Active Theory
12.2.13. Decentraland
12.2.14. Microsoft Corporation
12.2.15. Antier Solutions Pvt. Ltd.
12.2.16. Other Prominent Players
Pharmacovigilance Market By Service Provider Outlook-
Pharmacovigilance Market By Product Life Cycle Outlook-
Pharmacovigilance Market By Type Outlook-
Pharmacovigilance Market By Process Flow Outlook-
Pharmacovigilance Market By Therapeutic Area Outlook-
Pharmacovigilance Market By End Use Outlook-
Pharmacovigilance Market By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
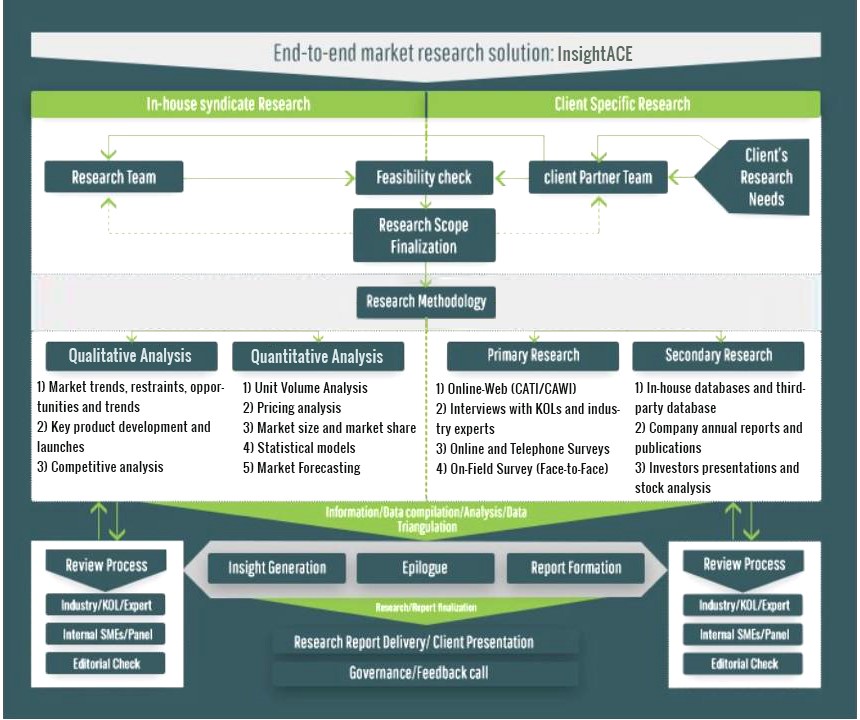
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.