Global Orphan Drugs CDMO Market Size is valued at USD 13.5 Bn in 2024 and is predicted to reach USD 27.6 Bn by the year 2034 at a 7.5% CAGR during the forecast period for 2025-2034.
Orphan drugs, also known as orphan medicines or orphan therapies, are pharmaceuticals designed to treat rare medical illnesses known as orphan diseases. These disorders often impact a small proportion of the population, making them commercially undesirable for drug companies to research and market in the absence of additional incentives. Several important drivers have influenced the Orphan Drugs CDMO (Contract Development and Manufacturing Organization) market, contributing to its growth and relevance in the pharmaceutical sector.
Orphan medications are in high demand due to increased awareness and diagnosis of rare diseases. As more uncommon diseases are discovered and characterized, the demand for specialized CDMOs to support their development and manufacture grows.However, the COVID-19 pandemic substantially impacted numerous sectors of the pharmaceutical business, particularly the CDMO (Contract Development and Manufacturing Organization) market for orphan drugs. The COVID-19 pandemic had a greater impact on specific pharmaceutical markets due to the deferral of non-life-threatening disease diagnosis and treatment. This was especially true for people suffering from uncommon diseases since various governments concentrated more financial and healthcare resources towards combating the pandemic.
The Orphan Drugs CDMO Market segmentation consists of drug type, therapy type, and end-user. As per the drug type, the market is segmented as Biologics and Non-biologics. The therapy type segment includes Oncology, Neuromuscular, Respiratory, Hematology, and Others. The end-user segment includes Pharmaceutical companies, Biotechnology companies, CROs, and Other End-users.
The Biologics category is expected to hold a major share of the global Orphan Drugs CDMO Market in 2022 due to the overwhelming prevalence of product offers classed as biologics. According to the U.S. FDA, the organization's Office of Orphan Products Development (OOPD) has developed and marketed approximately 600 medicines and biologic products to treat uncommon illnesses since 1983.
The personal care catagory is projected to grow at a rapid rate in the global Orphan Drugs CDMO Market. The Orphan Drugs Contract Development and Manufacture Organisation (CDMO) market assists pharmaceutical companies with orphan medication development and manufacture. The fundamental reason for the segment's dominance is that a considerable number of medications must be administered intravenously in hospitals by qualified healthcare workers.
The North America Orphan Drugs CDMO Market is projected to record the highest market share in terms of revenue in the near future. The substantial amount spent on orphan pharmaceuticals, the large patient population, and the existence of major market players all contributed to North America's dominance in the creation of sophisticated and cutting-edge technologies. For instance, the Genetic and Rare Diseases (GARD) Information Centre estimates that there are more than 10,000 rare diseases that afflict 30 million Americans, or about 1 in 10 persons, in the U.S. This contributes to North America's dominance in the worldwide market, together with the favourable reimbursement practices in the U.S. Due to a growing patient population in the region and a large uptake of cutting-edge treatments for rare diseases, the European market is expected to grow at a significant CAGR.
|
Report Attribute |
Specifications |
|
Market Size Value In 2024 |
USD 13.5 Bn |
|
Revenue Forecast In 2034 |
USD 27.6 Bn |
|
Growth Rate CAGR |
CAGR of 7.5% from 2025 to 2034 |
|
Quantitative Units |
Representation of revenue in US$ Bn,and CAGR from 2025 to 2034 |
|
Historic Year |
2021 to 2024 |
|
Forecast Year |
2025-2034 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Drug Type, Therapy Type, End-User |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; South East Asia; South Korea; South East Asia |
|
Competitive Landscape |
Novartis AG, F. Hoffmann-La Roche Ltd, Celgene, Bristol-Myers Squibb Company, Sanofi, Bayer Healthcare, Doppel, Lubrizol Life Science(LLS) Health, and Others |
|
Customization Scope |
Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing And Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global Orphan Drugs CDMO Market Snapshot
Chapter 4. Global Orphan Drugs CDMO Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. Competitive Landscape & Market Share Analysis
4.8. Impact of Covid-19 Analysis
Chapter 5. Market Segmentation 1: By Drug Type Estimates & Trend Analysis
5.1. By Drug Type, & Market Share, 2024 & 2034
5.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2021 to 2034 for the following By Drug Type:
5.2.1. Biologics
5.2.2. Non-biologics
Chapter 6. Market Segmentation 2: By Therapy Type Estimates & Trend Analysis
6.1. By Therapy Type & Market Share, 2024 & 2034
6.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2021 to 2034 for the following By Therapy Type:
6.2.1. Oncology
6.2.2. Neuromuscular
6.2.3. Respiratory
6.2.4. Hematology
6.2.5. Others
Chapter 7. Market Segmentation 3: By End User Estimates & Trend Analysis
7.1. By End User & Market Share, 2024 & 2034
7.2. Market Size (Value US$ Mn) & Forecasts and Trend Analyses, 2021 to 2034 for the following By End User:
7.2.1. Pharmaceutical companies
7.2.2. Biotechnology companies
7.2.3. CROs
7.2.4. Other End Users
Chapter 8. Orphan Drugs CDMO Market Segmentation 4: Regional Estimates & Trend Analysis
8.1. North America
8.1.1. North America Orphan Drugs CDMO Market revenue (US$ Million) estimates and forecasts By Drug Type, 2021-2034
8.1.2. North America Orphan Drugs CDMO Market revenue (US$ Million) estimates and forecasts By Therapy Type, 2021-2034
8.1.3. North America Orphan Drugs CDMO Market revenue (US$ Million) estimates and forecasts By End User, 2021-2034
8.1.4. North America Orphan Drugs CDMO Market revenue (US$ Million) estimates and forecasts by country, 2021-2034
8.2. Europe
8.2.1. Europe Orphan Drugs CDMO Market revenue (US$ Million) By Drug Type, 2021-2034
8.2.2. Europe Orphan Drugs CDMO Market revenue (US$ Million) By Therapy Type, 2021-2034
8.2.3. Europe Orphan Drugs CDMO Market revenue (US$ Million) By End User, 2021-2034
8.2.4. Europe Orphan Drugs CDMO Market revenue (US$ Million) by country, 2021-2034
8.3. Asia Pacific
8.3.1. Asia Pacific Orphan Drugs CDMO Market revenue (US$ Million) By Drug Type, 2021-2034
8.3.2. Asia Pacific Orphan Drugs CDMO Market revenue (US$ Million) By Therapy Type, 2021-2034
8.3.3. Asia Pacific Orphan Drugs CDMO Market revenue (US$ Million) By End User, 2021-2034
8.3.4. Asia Pacific Orphan Drugs CDMO Market revenue (US$ Million) by country, 2021-2034
8.4. Latin America
8.4.1. Latin America Orphan Drugs CDMO Market revenue (US$ Million) By Drug Type, (US$ Million) 2021-2034
8.4.2. Latin America Orphan Drugs CDMO Market revenue (US$ Million) By Therapy Type, (US$ Million) 2021-2034
8.4.3. Latin America Orphan Drugs CDMO Market revenue (US$ Million) By End User, (US$ Million) 2021-2034
8.4.4. Latin America Orphan Drugs CDMO Market revenue (US$ Million) by country, 2021-2034
8.5. Middle East & Africa
8.5.1. Middle East & Africa Orphan Drugs CDMO Market revenue (US$ Million) By Drug Type, (US$ Million) 2021-2034
8.5.2. Middle East & Africa Orphan Drugs CDMO Market revenue (US$ Million) By Therapy Type, (US$ Million) 2021-2034
8.5.3. Middle East & Africa Orphan Drugs CDMO Market revenue (US$ Million) By End User, (US$ Million) 2021-2034
8.5.4. Middle East & Africa Orphan Drugs CDMO Market revenue (US$ Million) by country, 2021-2034
Chapter 9. Competitive Landscape
9.1. Major Mergers and Acquisitions/Strategic Alliances
9.2. Company Profiles
9.2.1. Novartis AG
9.2.2. F. Hoffmann-La Roche Ltd
9.2.3. Celgene, Bristol-Myers Squibb Company
9.2.4. Sanofi
9.2.5. Bayer Healthcare
9.2.6. Doppel
9.2.7. Lubrizol Life Science (LLS) Health
9.2.8. Other Prominent Players
Orphan Drugs CDMO Market By Drug Type-
Orphan Drugs CDMO Market By Therapy Type-
Orphan Drugs CDMO Market By End-User-
Orphan Drugs CDMO Market By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
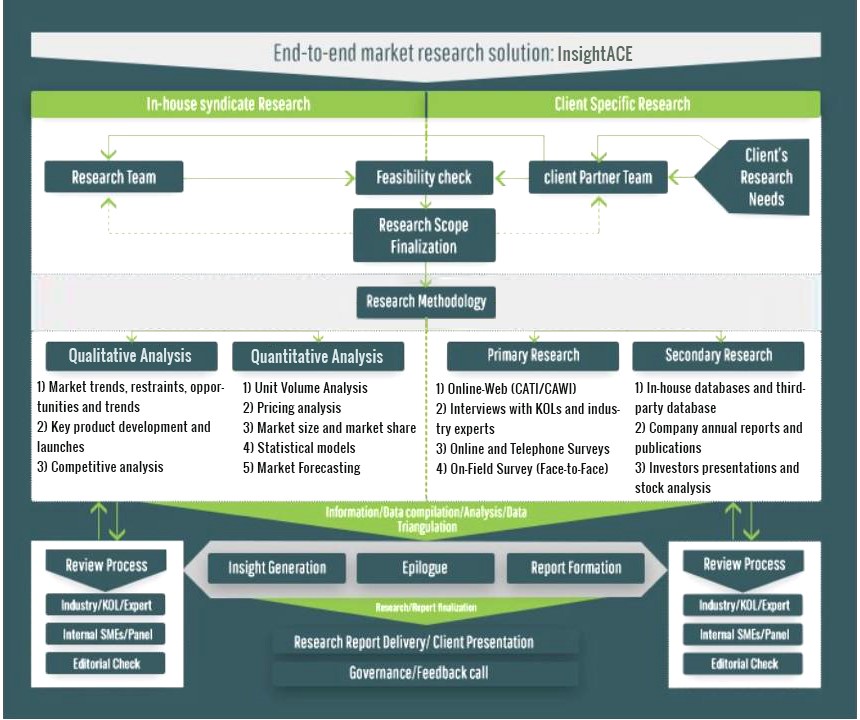
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.