In-vitro Transcription Templates Market Size is valued at USD 277.5 Mn in 2023 and is predicted to reach USD 1,134.1 Mn by the year 2031 at a 19.5% CAGR during the forecast period for 2024-2031.
In-vitro transcription templates are essential for a range of research and diagnostic purposes. These include creating mRNA for gene expression analysis, producing probes for RNA hybridization assays, and generating templates for in-vitro translation. In-vitro transcription templates are precise DNA sequences that act as templates for the production of RNA molecules through an in-vitro transcription process, which occurs outside of live cells. These templates include a promoter region that is recognized by RNA polymerase, which starts the process of transcribing the DNA sequence downstream into a complementary RNA strand.
There is a high need for in-vitro transcription templates due to the growing interest in RNA-based research, which encompasses gene expression studies, transcriptomics, and RNA medications. Researchers are actively investigating the potential of RNA molecules in various applications, fueling the expansion of the market. Furthermore, the rise in genetic abnormalities and developments in gene-editing technologies drive the need for in-vitro transcription templates.
However, the market growth is hampered by the high-cost criteria for the safety and health of the in-vitro transcription templates market and the product's inability to prevent fog in environments with dramatic temperature fluctuations or high in-vitro transcription templates because the exorbitant expense of R&D, which includes the creation of in-vitro transcription templates, can impede the expansion of the market. The process's high price tag makes it out of budget for smaller research labs and universities. In addition, Experts in molecular biology methods are needed in the area of in-vitro transcription. However, the lack of properly educated workers threatens the market's expansion and adoption. The market for in-vitro transcription templates has been affected in different ways by the COVID-19 epidemic. Even though the pandemic immediately hampered research and laboratory operations, it ultimately brought attention to the need for RNA-based research and therapies.
The in-vitro transcription templates market is segmented based on disease type, treatment, and end-user. Based on disease type, the market comprises cancer, infectious disease, lifestyle disease, genetic disease, and others. By treatment, the market is segmented into vaccine, therapeutic. The research stage segment includes exploratory and clinical. By end-user, the market is segmented into pharmaceutical & biotechnology companies, CROs & CMOs, academics & research, and others.
The therapeutic in-vitro transcription templates market will hold a major global market share in 2022. The use of activity as a therapeutic tool is a function-based approach that considers the patient's current and future functional abilities through environment-specific activities and task-grading. A solid therapeutic connection allows the client to take an active role in their recovery, improving results. Although interest in gene therapy and RNA-based treatments continues to grow, there will be a greater need for therapeutic in-vitro transcription templates.
The pharmaceutical & biotechnology companies make up the bulk of acrylic acid ester usage because the pharmaceutical and healthcare organizations work on topics such as RNA engineering and modification, therapeutic applications, synthetic biology, high-throughput screening, improved transcription efficiency, and template design and engineering, especially in countries like the US, Germany, the UK, China, and India.
The Asia Pacific in-vitro transcription templates market is expected to register the maximum market revenue share in the near future. It can be attributed to substantial investments in molecular biology research, a robust research infrastructure, and the presence of important market participants.
|
Report Attribute |
Specifications |
|
Market Size Value In 2023 |
USD 277.5 Mn |
|
Revenue Forecast In 2031 |
USD 1,134.1 Mn |
|
Growth Rate CAGR |
CAGR of 19.5% from 2024 to 2031 |
|
Quantitative Units |
Representation of revenue in US$ Mn and CAGR from 2024 to 2031 |
|
Historic Year |
2019 to 2023 |
|
Forecast Year |
2024-2031 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Disease Type, Treatment, Research Stage And End-Use |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; France; Italy; Spain; Southeast Asia; South Korea |
|
Competitive Landscape |
Thermo Fisher Scientific Inc., Promega Corporation, Agilent Technologies, Inc., New England Biolabs, Takara Bio Inc., LGC Limited, Enzynomics Co. Ltd., Enzo Life Sciences, Inc., and Danaher Corporation, Genscript Biotech Corporation, Eurofins Genomics LLC, TriLink Biotechnologies LLC, Bioline GMBH, Lucigen Corporation |
|
Customization Scope |
Free customization report with the procurement of the report and modifications to the regional and segment scope. Particular Geographic competitive landscape. |
|
Pricing And Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global In-vitro Transcription Templates Market Snapshot
Chapter 4. Global In-vitro Transcription Templates Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. Competitive Landscape & Market Share Analysis
4.8. Impact of Covid-19 Analysis
Chapter 5. Market Segmentation 1: by Disease Type Estimates & Trend Analysis
5.1. by Disease Type & Market Share, 2019 & 2031
5.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Disease Type:
5.2.1. Cancer
5.2.1.1. Solid Tumors
5.2.1.2. Colorectal Cancer
5.2.1.3. NSCLC
5.2.1.4. Melanoma
5.2.1.5. Leukemia
5.2.1.6. Prostate Cancer
5.2.1.7. Others
5.2.2. Infectious Disease
5.2.2.1. Influenza
5.2.2.2. COVID-19
5.2.2.3. AIDS
5.2.2.4. Others
5.2.3. Lifestyle Disease
5.2.4. Genetic Disease
5.2.5. Others
Chapter 6. Market Segmentation 2: by Treatment Estimates & Trend Analysis
6.1. by Treatment & Market Share, 2019 & 2031
6.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Treatment:
6.2.1. Vaccine
6.2.2. Therapeutic
Chapter 7. Market Segmentation 3: by Research Stage Estimates & Trend Analysis
7.1. by Research Stage & Market Share, 2019 & 2031
7.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Research Stage:
7.2.1. Exploratory
7.2.2. Clinical
Chapter 8. Market Segmentation 4: by End-user Estimates & Trend Analysis
8.1. by End-user & Market Share, 2019 & 2031
8.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by End-user:
8.2.1. Pharmaceutical & Biotechnology Companies
8.2.2. CROs & CMOs
8.2.3. Academics & Research
8.2.4. Others
Chapter 9. In-vitro Transcription Templates Market Segmentation 5: Regional Estimates & Trend Analysis
9.1. North America
9.1.1. North America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Disease Type, 2023-2031
9.1.2. North America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Treatment, 2023-2031
9.1.3. North America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Research Stage, 2023-2031
9.1.4. North America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by End-user, 2023-2031
9.1.5. North America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
9.2. Europe
9.2.1. Europe In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Disease Type, 2023-2031
9.2.2. Europe In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Treatment, 2023-2031
9.2.3. Europe In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Research Stage, 2023-2031
9.2.4. Europe In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by End-user, 2023-2031
9.2.5. Europe In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
9.3. Asia Pacific
9.3.1. Asia Pacific In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Disease Type, 2023-2031
9.3.2. Asia Pacific In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Treatment, 2023-2031
9.3.3. Asia-Pacific In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Research Stage, 2023-2031
9.3.4. Asia-Pacific In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by End-user, 2023-2031
9.3.5. Asia Pacific In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
9.4. Latin America
9.4.1. Latin America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Disease Type, 2023-2031
9.4.2. Latin America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Treatment, 2023-2031
9.4.3. Latin America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Research Stage, 2023-2031
9.4.4. Latin America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by End-user, 2023-2031
9.4.5. Latin America In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
9.5. Middle East & Africa
9.5.1. Middle East & Africa In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Disease Type, 2023-2031
9.5.2. Middle East & Africa In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Treatment, 2023-2031
9.5.3. Middle East & Africa In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by Research Stage, 2023-2031
9.5.4. Middle East & Africa In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by End-user, 2023-2031
9.5.5. Middle East & Africa In-vitro Transcription Templates Market Revenue (US$ Million) Estimates and Forecasts by country, 2023-2031
Chapter 10. Competitive Landscape
10.1. Major Mergers and Acquisitions/Strategic Alliances
10.2. Company Profiles
10.2.1. Thermo Fisher Scientific Inc.
10.2.2. Promega Corporation
10.2.3. Agilent Technologies, Inc.
10.2.4. New England Biolabs
10.2.5. Takara Bio Inc.
10.2.6. LGC Limited
10.2.7. Enzynomics Co. Ltd.
10.2.8. Enzo Life Sciences, Inc.
10.2.9. Danaher Corporation
10.2.10. Genscript Biotech Corporation
10.2.11. Eurofins Genomics LLC
10.2.12. TriLink Biotechnologies LLC
10.2.13. Bioline GMBH
10.2.14. Lucigen Corporation
10.2.15. Other Prominent Players
In-vitro Transcription Templates Market By Disease Type-
In-vitro Transcription Templates Market By Treatment
In-vitro Transcription Templates Market By Research Stage
In-vitro Transcription Templates Market By End-user
In-vitro Transcription Templates Market By Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
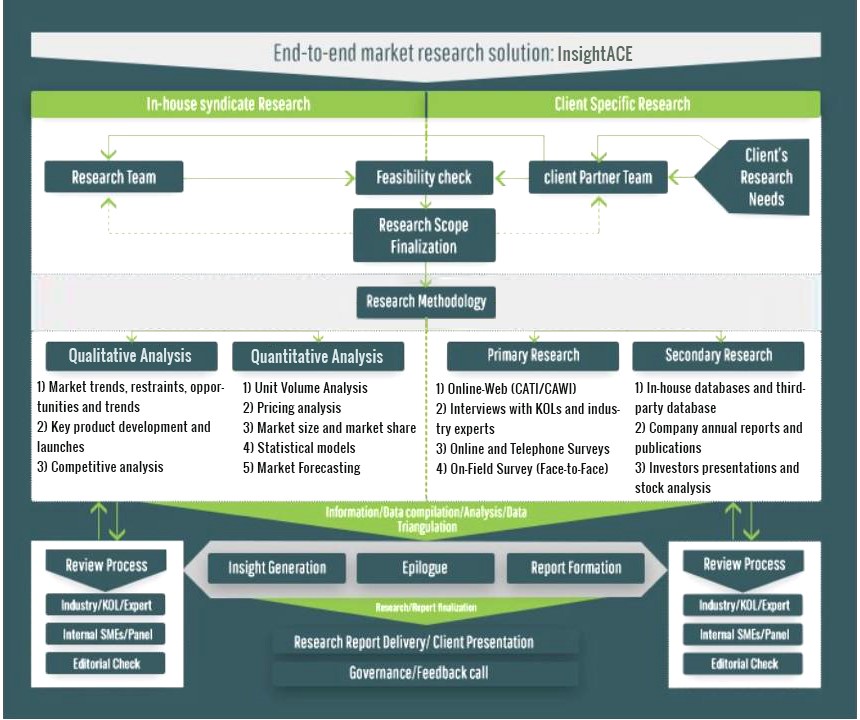
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.