The Clinical Trial Biorepository & Archiving Solutions Market Size is valued at USD 4.24 Bn in 2023 and is predicted to reach USD 8.49 Bn by the year 2031 at an 9.12% CAGR during the forecast period for 2024-2031.
Clinical trial biorepositories and archiving solutions play pivotal roles in the pharmaceutical and biomedical research sectors by providing specialized facilities and technologies for the storage, processing, and management of biological samples and clinical trial data. Biorepository services encompass the storage, management, and preservation of various biological specimens such as blood, tissue, and bodily fluids. These facilities maintain the integrity and long-term viability of samples, ensuring they are accessible for future research and development efforts aimed at treating diverse diseases. By offering well-characterized samples, biorepositories facilitate collaborative research initiatives among scientists and pharmaceutical companies, thereby accelerating the discovery of biomarkers and the development of innovative therapeutics.
Simultaneously, archiving solution services manage the vast quantities of data generated during clinical trials. These solutions organize and preserve data in a systematic manner, ensuring regulatory compliance and enabling researchers to extract valuable insights. Effective data management and integration empower researchers to analyze large datasets comprehensively, identify trends, and derive meaningful conclusions from patient information.
The Clinical Trial Biorepository & Archiving Solutions market is driven by increasing pharmaceutical R&D activities and significant technological advancements. These include enhanced data management systems, improved sample storage and retrieval processes, and innovations in cryopreservation and automated sample handling. These factors collectively boost efficiency, support regulatory compliance, and foster collaboration, thereby advancing biomedical research and drug development capabilities.
The clinical trial biorepository & archiving solutions market is segmented based on service, product and phase. By service the market is segmented into biorepository services and archiving solution services, biorepository services is sub segmented into warehousing & storage, transportation, sample processing, others, archiving solution services is sub segmented into database indexing and management, scanning & destruction. By product the market is segmented into preclinical products and clinical products, clinical products is sub segmented into human tissue, organs, stem cells, and other biospecimens. By phase the market is segmented into phase i, phase ii, phase iii, phase iv.
The Biorepository Services segment stands out in the Clinical Trial Biorepository and Archiving Solutions market due to increasing demand driven by oncology research and personalized medicine. These services provide essential biological samples for biomarker discovery, treatment response analysis, and targeted therapy development, crucial for advancing medical research. Technological advancements in automated sample handling, cryopreservation, and data management systems further enhance their efficiency and effectiveness. Compliance with regulations such as HIPAA ensures patient privacy and data security, solidifying biorepository services as pivotal in supporting collaborative research efforts and enabling data-driven insights in biomedical research and therapeutic innovation.
The Preclinical segment is experiencing rapid growth within the Clinical Trial Biorepository and Archiving Solutions market, driven by several key factors. Foremost among these is the accelerated approval rate of novel drugs, vaccines, and medicines by regulatory bodies, which spurs demand for preclinical research services. The segment benefits from an increased success rate in preclinical trials and reduced production costs, making it increasingly attractive for pharmaceutical and biotechnology companies. Biorepository services play a crucial role in this growth by providing secure and controlled environments for the storage and management of biological specimens essential for preclinical trials.
North America is at the forefront of the Clinical Trial Biorepository and Archiving Solutions market, driven by several key factors. The region hosts a robust pharmaceutical industry characterized by a high volume of clinical trials aimed at developing new treatments and therapies. Additionally, North America boasts a dense network of research institutions, universities, and hospitals that actively conduct clinical trials, necessitating reliable biorepository and archiving solutions for the storage and management of biological samples and trial data. Furthermore, substantial investments in pharmaceutical research and development by companies in the region, coupled with government funding initiatives like those from the National Institutes of Health (NIH), further bolster the demand for these solutions.
|
Report Attribute |
Specifications |
|
Market Size Value In 2023 |
USD 4.24 Bn |
|
Revenue Forecast In 2031 |
USD 8.49 Bn |
|
Growth Rate CAGR |
CAGR of 9.12% from 2024 to 2031 |
|
Quantitative Units |
Representation of revenue in US$ Bn and CAGR from 2024 to 2031 |
|
Historic Year |
2019 to 2023 |
|
Forecast Year |
2024-2031 |
|
Report Coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments Covered |
By Service, By Product, By Phase, and By Region |
|
Regional Scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country Scope |
U.S.; Canada; U.K.; Germany; China; India; Japan; Brazil; Mexico; The UK; France; Italy; Spain; China; Japan; India; South Korea; Southeast Asia; South Korea; Southeast Asia |
|
Competitive Landscape |
Azenta U.S., Inc., Thermo Fisher Scientific Inc. (Patheon), Precision for Medicine, Inc., Medpace, LabCorp Drug Development, ATCC, Q2 Solutions, Labconnect, Charles River Laboratories, Cell&Co, Other Prominent Players |
|
Customization Scope |
Free customization report with the procurement of the report, Modifications to the regional and segment scope. Geographic competitive landscape. |
|
Pricing and Available Payment Methods |
Explore pricing alternatives that are customized to your particular study requirements. |
Chapter 1. Methodology and Scope
1.1. Research Methodology
1.2. Research Scope & Assumptions
Chapter 2. Executive Summary
Chapter 3. Global Clinical Trial Biorepository & Archiving Solutions Market Snapshot
Chapter 4. Global Clinical Trial Biorepository & Archiving Solutions Market Variables, Trends & Scope
4.1. Market Segmentation & Scope
4.2. Drivers
4.3. Challenges
4.4. Trends
4.5. Investment and Funding Analysis
4.6. Industry Analysis – Porter’s Five Forces Analysis
4.7. Competitive Landscape & Market Share Analysis
4.8. Impact of Covid-19 Analysis
Chapter 5. Market Segmentation 1: by Service Estimates & Trend Analysis
5.1. by Service & Market Share, 2023 & 2031
5.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Service:
5.2.1. Biorepository Services
5.2.1.1. Warehousing & Storage
5.2.1.2. Transportation
5.2.1.3. Sample Processing
5.2.1.4. Others
5.2.2. Archiving Solution Services
5.2.2.1. Database Indexing and Management
5.2.2.2. Scanning & Destruction
Chapter 6. Market Segmentation 2: by Product Estimates & Trend Analysis
6.1. by Product & Market Share, 2023 & 2031
6.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Product:
6.2.1. Preclinical Products
6.2.2. Clinical Products
6.2.2.1. Human Tissue
6.2.2.2. Organs
6.2.2.3. Stem Cells
6.2.2.4. Other Biospecimens
Chapter 7. Market Segmentation 3: by Phase Estimates & Trend Analysis
7.1. by Phase & Market Share, 2023 & 2031
7.2. Market Size (Value (US$ Mn)) & Forecasts and Trend Analyses, 2019 to 2031 for the following by Phase:
7.2.1. Phase I
7.2.2. Phase II
7.2.3. Phase III
7.2.4. Phase IV
Chapter 8. Clinical Trial Biorepository & Archiving Solutions Market Segmentation 4: Regional Estimates & Trend Analysis
8.1. North America
8.1.1. North America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Service, 2024-2031
8.1.2. North America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Product, 2024-2031
8.1.3. North America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Phase, 2024-2031
8.1.4. North America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by country, 2024-2031
8.2. Europe
8.2.1. Europe Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Service, 2024-2031
8.2.2. Europe Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Product, 2024-2031
8.2.3. Europe Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Phase, 2024-2031
8.2.4. Europe Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by country, 2024-2031
8.3. Asia Pacific
8.3.1. Asia Pacific Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Service, 2024-2031
8.3.2. Asia Pacific Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Product, 2024-2031
8.3.3. Asia-Pacific Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Phase, 2024-2031
8.3.4. Asia Pacific Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by country, 2024-2031
8.4. Latin America
8.4.1. Latin America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Service, 2024-2031
8.4.2. Latin America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Product, 2024-2031
8.4.3. Latin America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Phase, 2024-2031
8.4.4. Latin America Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by country, 2024-2031
8.5. Middle East & Africa
8.5.1. Middle East & Africa Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Service, 2024-2031
8.5.2. Middle East & Africa Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Product, 2024-2031
8.5.3. Middle East & Africa Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by Phase, 2024-2031
8.5.4. Middle East & Africa Clinical Trial Biorepository & Archiving Solutions Market Revenue (US$ Million) Estimates and Forecasts by country, 2024-2031
Chapter 9. Competitive Landscape
9.1. Major Mergers and Acquisitions/Strategic Alliances
9.2. Company Profiles
9.2.1. Azenta U.S., Inc.
9.2.2. Thermo Fisher Scientific Inc. (Patheon)
9.2.3. Precision for Medicine, Inc.
9.2.4. Medpace
9.2.5. LabCorp Drug Development
9.2.6. ATCC
9.2.7. Q2 Solutions
9.2.8. Labconnect
9.2.9. Charles River Laboratories
9.2.10. Cell&Co
9.2.11. Other Prominent Players
Clinical Trial Biorepository & Archiving Solutions Market by Solutions-
Clinical Trial Biorepository & Archiving Solutions Market by Product-
Clinical Trial Biorepository & Archiving Solutions Market by Phase-
Clinical Trial Biorepository & Archiving Solutions Market by Region-
North America-
Europe-
Asia-Pacific-
Latin America-
Middle East & Africa-
InsightAce Analytic follows a standard and comprehensive market research methodology focused on offering the most accurate and precise market insights. The methods followed for all our market research studies include three significant steps – primary research, secondary research, and data modeling and analysis - to derive the current market size and forecast it over the forecast period. In this study, these three steps were used iteratively to generate valid data points (minimum deviation), which were cross-validated through multiple approaches mentioned below in the data modeling section.
Through secondary research methods, information on the market under study, its peer, and the parent market was collected. This information was then entered into data models. The resulted data points and insights were then validated by primary participants.
Based on additional insights from these primary participants, more directional efforts were put into doing secondary research and optimize data models. This process was repeated till all data models used in the study produced similar results (with minimum deviation). This way, this iterative process was able to generate the most accurate market numbers and qualitative insights.
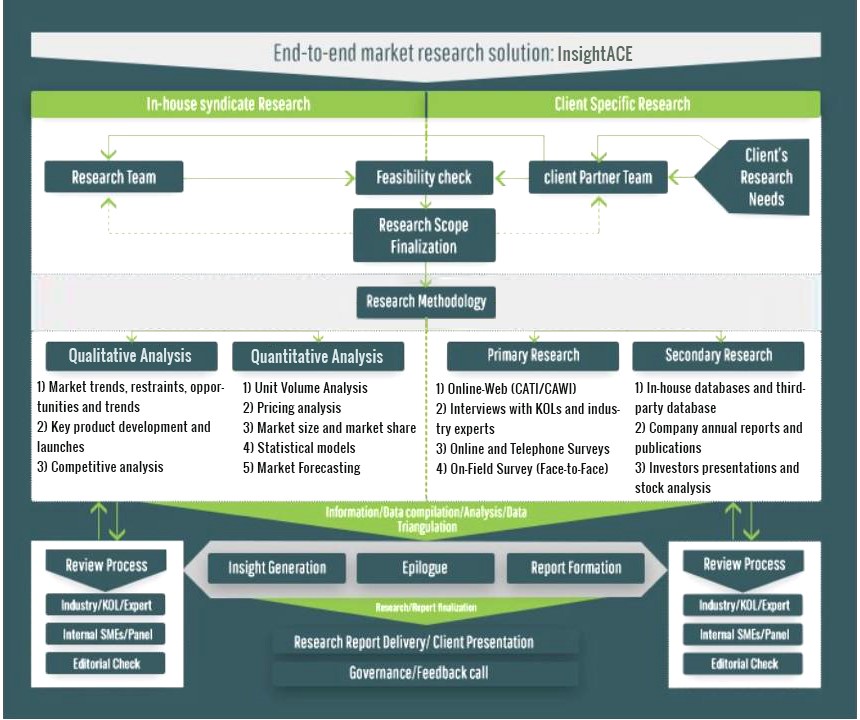
Secondary research
The secondary research sources that are typically mentioned to include, but are not limited to:
The paid sources for secondary research like Factiva, OneSource, Hoovers, and Statista
Primary Research:
Primary research involves telephonic interviews, e-mail interactions, as well as face-to-face interviews for each market, category, segment, and subsegment across geographies
The contributors who typically take part in such a course include, but are not limited to:
Data Modeling and Analysis:
In the iterative process (mentioned above), data models received inputs from primary as well as secondary sources. But analysts working on these models were the key. They used their extensive knowledge and experience about industry and topic to make changes and fine-tuning these models as per the product/service under study.
The standard data models used while studying this market were the top-down and bottom-up approaches and the company shares analysis model. However, other methods were also used along with these – which were specific to the industry and product/service under study.
To know more about the research methodology used for this study, kindly contact us/click here.